Obesity, particularly abdominal obesity, is currently considered a chronic low-grade inflammatory condition that plays an active role in the development of the pathophysiologic phenomena responsible for metabolic syndrome and cardiovascular disease through the secretion of proinflammatory adipokines and cytokines. In recent years clear genetic, pathogenic, and epidemiologic links have been established between psoriasis and obesity, with important implications for health. The relationship between the 2 conditions is probably bidirectional, with obesity predisposing to psoriasis and psoriasis favoring obesity.
Obesity also has important implications in the treatment of psoriasis, such as a greater risk of adverse effects with conventional systemic drugs and reduced efficacy and/or increased cost with biologic agents, for which dosage should be adjusted to the patient's weight.
La obesidad, en particular la abdominal, se considera en la actualidad como un proceso inflamatorio crónico de bajo grado que participa de forma activa en el desarrollo de los fenómenos fisiopatológicos responsables del síndrome metabólico y la morbilidad cardiovascular a través de la secreción de adipocinas y citocinas proinflamatorias. En los últimos años se ha establecido un vínculo firme entre psoriasis y obesidad que abarca aspectos genéticos, patogénicos y epidemiológicos, con importantes repercusiones en la salud del individuo. Es probable una relación bidireccional, en la que la obesidad predispone a la psoriasis, pero también la psoriasis favorece la obesidad.
La obesidad tiene también importantes implicaciones terapéuticas, como el mayor riesgo de efectos adversos en el caso de los fármacos sistémicos convencionales y la disminución de la eficacia y/o el incremento del coste en el caso de los fármacos biológicos, que hace recomendable ajustar la dosis al peso del paciente.
Obesity is defined as a chronic condition characterized by excess weight due to an increase in energy deposits stored as body fat. A defect in the symbiosis and equilibrium of food intake associated with a deregulation of energy expenditure, whether due to inflammatory or genetic disease or caused by excesses or alterations in food intake, will lead to a state of obesity or thinness.1
A diagnosis of obesity is established by determining the patient's body mass index (BMI) using the formula weight in kilograms divided by the square of the height in meters. The current World Health Organization weight classification for BMI in adults is as follows: a BMI of between 18.5 and 24.9 is normal, 25 to 29.9 is overweight, and a BMI greater than 30 is diagnostic of obesity.2,3
The establishment in recent years of a clear association between psoriasis and obesity coincided with a growing awareness that both diseases are chronic inflammatory processes that have significant repercussions on the individual's health, largely in terms of increased risk of cardiovascular disease and the elements of metabolic syndrome. Although there is still considerable debate about this association with psoriasis, the evidence points to a bidirectional relationship. Several epidemiological studies have provided evidence supporting the hypothesis that obesity is an independent risk factor associated with a high risk of psoriasis and a poor long-term prognosis in that setting; however, the findings of other studies appear to indicate that obesity may be a consequence of psoriasis rather than a risk factor for the condition.4–6
The aim of the present review is to provide a general analysis of the relationship between psoriasis and obesity. Starting with an account of the currently accepted view of the inflammatory nature of obesity, we will review the relationship between psoriasis and obesity and the pathogenic basis of this association. Finally, we will discuss the challenges and implications of the association in the treatment of psoriasis.
Inflammatory Nature of ObesityBefore the first adipokine—leptin—was discovered in 1994, it was thought that the only role of adipose tissue (AT) was to store energy in the form of fat; however, it is now clear that AT is an endocrine, autocrine, and paracrine organ that performs other functions in addition to storing energy reserves.7
AT, the largest organ in the adult human body, is composed of adipocytes, cells that are responsible for storing energy in the form of triglycerides. Recent evidence indicates that AT, and especially abdominal fat, is an active endocrine organ which helps to regulate several body functions, including insulin-mediated processes, lipid and glucose metabolism, and vascular biology, and plays a role in coagulation and some aspects of inflammation.8,9
All these effects are mediated by various adipokines—regulators secreted by adipocytes—and a wide variety of proinflammatory cytokines, including C-reactive protein (CRP), transforming growth factor β, plasminogen activator inhibitor-1 (PAI-1), interleukin (IL) 1β, IL-6, and tumor necrosis factor-alpha (TNF-α). The direct and indirect effects of these molecules are the key to the inflammatory nature of obesity and its relationship with other inflammatory processes including psoriasis.
In obesity, the structure and composition of AT is altered and these changes enhance the proinflammatory effect. Leptin, and possibly other factors produced by adipocytes and macrophages, upregulate or markedly increase endothelial cell adhesion molecules, such as intercellular adhesion molecule-1 (ICAM-1) and platelet endothelial cell adhesion molecule-1 (PECAM-1). It is also possible that monocyte-chemoattractant molecule-1 (MCP-1), a chemokine expressed in adipocytes that can be correlated with body weight, contributes to the recruitment and transmigration of bone marrow–derived monocytes, thereby producing an increase in macrophages in AT. Fusions with resident macrophages would generate multinucleated giant cells. It is proposed that this accumulation of macrophages, which are increased in both number and size and may account for as much as 60% of cells depending on the patient's fat mass, triggers the expression of proinflammatory molecules, thereby contributing to the ongoing inflammatory state.
Another important finding is that lymphocytes, which do not form part of the adipose tissue, are often found in close proximity to the adipocytes surrounding the lymph node; consequently, there may be paracrine relationships between the lymphocytes and adipocytes, allowing the exchange of information between the two.10,11
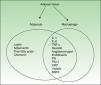
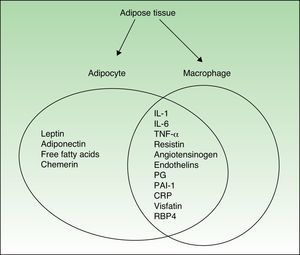
Figure 1 shows the cytokines secreted by activated macrophages and adipocytes present in AT. In fact, some authors consider AT to be part of the innate immune system.12
In addition to the cells mentioned above, AT also contains endothelial cells, leukocytes, fibroblasts, and preadipocytes, which make up the adipose stromal vascular tissue.
These are the cells that make up AT, which is regulated by multiple metabolic pathways mediated by cytokines (produced in the AT or elsewhere) and by adipokines (also called adipocytokines). Adipokines, which are a bioactive product of AT with endocrine, paracrine, and autocrine effects, are key players in the proinflammatory state associated with obesity.13,14
The presence and persistence of a low-grade inflammatory state in patients with chronic obesity results in impaired vascular structure and function as well as alterations in immune regulation and adipocyte metabolism caused by alterations in the equilibrium between adipokines and cytokines, which play a key role in the pathogenesis of metabolic syndrome and the risk of cardiovascular morbidity and mortality.
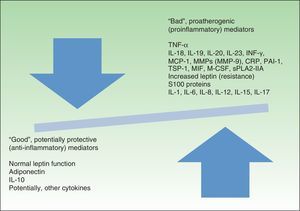
Figure 2 presents a modified version of Fisman's classification of the molecules associated with inflammation that affect cardiovascular risk.
Favorable and unfavorable mediators of cardiovascular risk.IL indicates interleukin; TNF, tumor necrosis factor; INF-γ, interferon-γ; MCP-1, monocyte-chemoattractant protein 1; MMP, matrix metalloproteinase; CRP, C-reactive protein; PAI-1, plasminogen activator inhibitor-1; TSP-1, thrombospondin-1; MIF, migration inhibitory factor; M-CSF, macrophage colony-stimulating factor; sPLA2-IIA, secretory phospholipase A2 group IIA.
In the following sections, we will describe the molecules involved in the inflammatory state associated with obesity.
AdipokinesAT, and principally visceral AT, is an immune-metabolic-endocrine organ and, as such, is capable of secreting substances with endocrine, paracrine, and autocrine activity collectively termed adipokines.15
Adipokines can induce obesity (leptin), insulin resistance (resistin), inflammation, dyslipidemia, hypercoagulability, and endothelial dysfunction associated with arteriosclerosis; all of these elements are involved in the onset and progression of metabolic syndrome. By contrast, some adipokines, such as adiponectin, may exert a protective effect against metabolic syndrome.
AdiponectinAdiponectin is produced primarily by adipocytes. The gene that codes for adiponectin is located in the region associated with genetic susceptibility to metabolic syndrome, type 2 diabetes mellitus, and cardiovascular disease.16 There is an inverse relationship between adiponectin levels and BMI.17 In obesity, adiponectin is decreased, due partly to an increase in proinflammatory cytokines and adipokines such as TNF-α and IL-6.18 Adiponectin has an important anti-inflammatory role because it induces the secretion of IL-10 and IL-1 receptor antagonist in monocytes and macrophages and inhibits the production of TNF-α, IL-6, and ICAM-1. Low plasma concentrations of adiponectin have been associated with an excess of visceral AT in patients with dyslipidemia, insulin resistance, cardiovascular disease, and hypertension.19
Adiponectin levels are variable in chronic inflammatory processes. Levels may be elevated in inflammatory processes unrelated to obesity or excessive visceral AT, such as rheumatoid arthritis; the opposite may occur in chronic diseases characterized by an excess of visceral fat and obesity, such as psoriasis, type 2 diabetes, and metabolic syndrome, in which adiponectin levels are greatly reduced.20
LeptinWhile leptin is produced primarily by adipocytes, it is also expressed in other tissues, such as the placenta, ovaries, skeletal muscle, stomach, pituitary gland, and liver.21,22
Leptin signals whether somatic reserves of fat deposits are sufficient for growth and reproduction. Low leptin levels are indicative of insufficient energy reserves, a situation that gives rise to hyperphagia, low energy expenditure, and infertility since the body is not prepared for pregnancy. However, a serum leptin concentration above the signaling threshold may have little or no physiological effect on hypophagia, demonstrating that adipokines do not act alone and that interplay between them involving many different combinations is needed to produce a particular effect on weight, inflammation, etc.23
Leptin-deficient patients are extremely obese and children with congenital leptin deficiency remain prepubertal if not treated with leptin replacement therapy.24,25
High levels of leptin have been associated with cardiovascular disorders and some authors have suggested that leptin is an independent predictor of such cardiovascular events and coronary heart disease. Studies in mice have also shown that leptin accelerates thrombus formation in blood vessels that have been damaged by inflammation.26–30
Leptin also has a strong immunomodulatory effect.20 Studies have shown that, a T helper 1 response is produced when mononucleated cells from peripheral human serum are incubated with high doses of leptin; this finding supports the hypothesis that leptin is involved in the regulation of inflammatory processes.31,32
Some authors suggest that leptin could serve as a marker of the severity and chronicity of psoriasis. Leptin may stimulate angiogenesis and keratinocyte proliferation and, in conjunction with obesity, may predispose patients to psoriasis.9,33,34
ResistinThe adipose-derived adipokine resistin is associated primarily with inflammation, immunity, obesity, and insulin resistance. It is produced by the monocytes and macrophages of AT and peripheral blood. Increased resistin expression, and consequently inflammation, may be a mediator of endothelial dysfunction and an early warning sign for arteriosclerosis.35,36
Proinflammatory cytokines, such as TNF-α, IL-1-β, IL-6, and lipopolysaccharides, can increase resistin expression, which in turn can upregulate the production of TNF-α and IL-12.36
Although resistin was initially characterized in humans as a link between obesity and diabetes, this hypothesis has not been satisfactorily demonstrated because of the inconsistent results of different studies.
Resistin levels have been shown to be predictive of coronary heart disease and markers of the severity of ischemic cardiac damage.37,38
Retinol-Binding Protein 4Retinol-binding protein 4 (RBP4), the serum transport protein for vitamin A, is produced and secreted by hepatocytes and primarily by visceral adipocytes.39 Elevated RBP4 levels have been linked to obesity and insulin resistance. A high RBP4 level before the onset of diabetes indicates a high degree of insulin resistance and the need to further adjust treatment.40–42 RBP4 has been linked directly to cardiovascular disease associated with obesity, but not with psoriasis.
OmentinOmentin is a protein mainly produced by the stromal and vascular cells of visceral AT. It enhances sensitivity to insulin and has been shown to be involved in the pathogenesis of obesity and related diseases. Souza et al.43found omentin levels to be inversely correlated with obesity and concluded that high levels of this protein could be a marker of protection against obesity.44
ChemerinChemerin is a protein that regulates adipocyte development and metabolic functions, such as hepatic and muscle glucose metabolism. Chemerin levels are elevated in obese patients and correlate positively with several components of metabolic syndrome. The dual role of this adipokine in inflammation and metabolism connects chronic inflammation and obesity, and also provides a link to associated diseases, such as type 2 diabetes and cardiovascular disease.45
LipocalinThe role of lipocalin in obesity is still not clearly defined. Lipocalin levels have been shown to correlate with other adipokines, positively in the case of TNF-α and IL-6 levels. They also correlate positively with the diameter of adipocytes in visceral AT.46
α-Melanocyte-Stimulating Hormoneα melanocyte-stimulating hormone (α-MSH) is, together with ghrelin and leptin, a key regulator of energy balance. It has effects on the central nervous system, the immune system, and inflammation. It inhibits food intake by stimulating the melanocortin receptor in the brain. Certain variants of the melanocortin 4 receptor gene (MC4R) are associated with abnormalities in the patient's weight and when the melanocortin receptor in the hypothalamus is missing or dysfunctional massive obesity will result. Heterozygous mutation of MC4R has been shown to be the leading cause of severe obesity in children. Some studies have shown that α-MSH plays a more important role than leptin in the regulation of food intake and energy expenditure. However, in growth and other endocrine axes leptin is more active and plays a more important role.
Levels of both leptin and α-MSH are elevated in obese patients, although α-MSH has the opposite effect to leptin with respect to the inflammatory response because it reduces the secretion of prostaglandins, thereby reversing the effect of TNF-α on melanocytes.47
ApelinApelin is a bioactive peptide produced by adipocytes, stromal cells, the heart, and the cardiovascular system. In humans, obesity and increased insulin levels upregulate apelin. This peptide appears to act as a circulating paracrine hormone and its receptor has some of the same characteristics as the angiotensin receptor. For example, apelin also regulates insulin resistance and influences circulating levels of adiponectin. In rats, apelin has been reported to have a positive hemodynamic effect and to act as an inotrope.48,49
VaspinIn humans, vaspin is expressed in visceral and subcutaneous AT. It has been shown to specifically regulate fat deposits and to be linked with obesity and insulin resistance. Elevated vaspin levels are associated with obesity and insulin sensitivity.50–53
Free Fatty AcidsFree fatty acids are considered to be an important link between chronic inflammation and AT activity, since they increase oxidative stress, thereby inducing inflammation and impairing vascular reactivity.54
Plasminogen Activator Inhibitor-1PAI-1 is secreted by AT, the liver, and endothelial tissue. Levels are elevated in obese and insulin-resistant patients and are associated with the induction of metabolic syndrome. High levels of PAI-1 are also predictive of an increased risk of type 2 diabetes and cardiovascular problems. Thus, PAI-1 may contribute to the development of obesity and insulin resistance and is potentially a link between obesity and cardiovascular disease.55
CytokinesTumor Necrosis FactorTNF is a proinflammatory molecule present in the bloodstream as a soluble molecule; it is produced by a variety of cells, including activated monocytes and macrophages, lymphocytes, mast cells, and natural killer cells. In obesity, TNF-α is produced mainly by the macrophages of stromal and vascular AT.
It has been shown that messenger RNA (mRNA) expression of TNF-α is elevated in the adipocytes of genetically obese mice56 and in samples of human AT from patients with a high percentage of body fat (r = 0.46).57 The expression of TNF-α mRNA and TNF-α protein are 2.5 and 2 times greater, respectively, in the adipocytes of obese individuals relative to normal-weight controls.58 Similarly, the expression of TNF-α in the adipocytes of obese patients decreases when the patient loses weight. The evidence currently available indicates that circulating levels of human TNF-α receptors are elevated in obesity.59 However, we should note that high plasma levels have not been observed in obese patients in the majority of studies. Therefore, most experts believe that the proadipogenic effects of TNF-α are the result of direct autocrine or paracrine actions occurring within the AT.58,59
TNF-α enhances its own production and that of IL-6, resistin, visfatin, and MCP-1.60–62 By contrast, it downregulates the levels of adiponectin and leptin produced by adipocytes.63
TNF-α also contributes to insulin resistance through the induction of serine phosphorylation of the insulin receptor substrate 1, which in turn reduces the tyrosine kinase activity of the insulin receptor.64–66 Treatment of obese rats with soluble TNF-α receptors normalizes the expression of TNF-α itself and enhances insulin sensitivity.56 This phenomenon has also been demonstrated passively: the administration of exogenous TNF-α reduces insulin sensitivity in healthy animals.67 All of these findings indicate that TNF-α may have a lipostatic function. However, paradoxically, the findings of Domínguez et al.68 in a study of 20 obese patients with insulin-resistant diabetes showed that blocking TNF-α with etanercept did not significantly increase insulin sensitivity despite a reduction in serum levels of inflammatory cytokines such as IL-6 and CRP.
An in vitro study of TNF and AT cells showed that the formation of proinflammatory cytokines was neutralized when TNF-α was inhibited. The authors concluded that TNF-α could play a crucial role in the regulation of adipokines in adipocytes.69–71
Interleukin-6IL-6, considered to be one of the most important inflammatory mediators, has both proinflammatory and anti-inflammatory effects. It has also been shown to regulate hematopoiesis, the immune response, and inhibition of defense mechanisms.72
IL-6 is produced by fibroblasts, endothelial cells, monocytes, and adipocytes.71
Some 30% of circulating IL-6 originates in stromal AT.73 Very high IL-6 levels are found in episodes of severe stress, surgery, sepsis, and high levels are observed in obesity.73 In obese patients, the expression and excretion of IL-6 derived from visceral AT correlates directly with increases in BMI and AT. The release of IL-6 into the bloodstream has been linked to insulin resistance and found to be a predictor of type 2 diabetes.42
Studies in mice have shown that a diet rich in free fatty acids induces an increase in IL-6 secreted by AT and induces insulin resistance in the liver.74
Other effects of IL-6 include the upregulation of acute phase reactants, such as CRP, PAI-1, and fibrinogen. In this way, IL-6 is another important link between AT and inflammation.75
Other cytokines involved in this association are IL-1, IL-5, MCP-1, and macrophage colony-stimulating factor.
Inflammatory Nature of Obesity: ConclusionsIt has recently been shown that, far from being just a passive energy store, AT is a highly active metabolic and endocrine organ. It is also now known that obesity is in itself a low-grade chronic inflammatory process in which one of the key cell components is the AT macrophage. Furthermore, there is now sufficient scientific evidence to support the assertion that the inflammation inherent in obesity plays an active role in the development of the pathophysiological phenomena responsible for metabolic syndrome, cardiovascular disease, and psoriasis. Based on the results of population-based studies, certain parameters of chronic inflammation have been postulated as markers of cardiovascular risk. These include elevated serum levels of CRP, IL-6, TNF-α, and leptin as well as reduced levels of adiponectin and IL-10. These 2 last correlate particularly with the elements of metabolic syndrome, including obesity.
Psoriasis and ObesityThe association between obesity and psoriasis has been the subject of several review articles and is a topic of growing interest.76–79 The first reference to this relationship was in a Swedish study of some 159 200 individuals who were followed up over a 10-year period.5 The association has since been corroborated by numerous more recent studies.4,80–86 The authors of an Italian case-control study of 560 patients with psoriasis found that the risk of psoriasis in an overweight (BMI 26-29) or obese (BMI ≥ 30) population was higher than in a nonobese control group (odds ratio [OR], 1.6 and 1.9, respectively).80 In the United Kingdom, Neimann et al.81 studied 127 706 patients with mild psoriasis (defined as disease not treated with systemic therapy or phototherapy) and 3854 patients with severe psoriasis (defined as disease treated with systemic therapy or phototherapy). They found a higher probability of obesity among patients with severe psoriasis (OR,1.8) than among those with mild psoriasis (OR,1.3) and patients without psoriasis.81 This tendency was confirmed in another study involving 16 851 patients with psoriasis, in which psoriatic patients under 35 years of age were shown to be more likely to be obese (OR,2.2) than those aged over 65 years (OR,1.6) relative to healthy controls.82 Overall, the findings of these studies suggest that a positive correlation exists between body weight and the prevalence and severity of psoriasis.
Which Comes First, Obesity or psoriasis?The epidemiological evidence currently available is insufficient to establish which comes first, obesity or psoriasis.
Herron et al.83 asked 557 psoriatic patients to recall their body size when they were 18 years of age (in all cases prior to the onset of psoriasis) and also assessed the patients’ current weight (after the onset of psoriasis). By this rather curious retrospective method, they ascertained that the patients who reported being obese at 18 years of age did not have an increased risk for psoriasis. However, the patients who subsequently developed psoriasis did have a higher propensity to obesity, a finding that suggests that the psoriasis preceded the appearance of obesity. When Mallbris et al.87 compared 200 patients within 12 months of onset of psoriasis, differentiating between a normal BMI and values in the obese range, they found no statistically significant differences in BMI between the 2 groups. In fact, although a tendency toward obesity was found in both groups, this was somewhat higher in the control group (13%) than in the psoriasis group (10%).
Various mechanisms have been proposed to explain how psoriasis might lead to obesity. These include progressive social isolation, poor eating habits, depression, increased alcohol consumption, and decreased physical activity (more pronounced in patients with psoriatic arthritis). In a case-control study, Zamboni et al.88 observed that both men and women with psoriasis consumed significantly more total fat, saturated fat, and alcohol than healthy controls. Furthermore, only 43% of the obese patients with psoriasis in the study by Herron et al.83 exercised 30 minutes or more at least 2 or 3 times a week, compared with 59% of the nonobese patients with psoriasis. Those authors also found that the percentage of obese patients who said that arthritis prevented them from engaging in physical activity was double that of nonobese patients.
Despite the findings of the studies cited above, another, conflicting, hypothesis has been proposed, which is that obesity predisposes patients to psoriasis. The earliest evidence to support this theory comes from reports on the health of prisoners of war who developed malnutrition during World War II. The reports indicated that psoriasis improved with weight loss and a decrease in calorie intake.89 More recently, Rucevic et al.90 demonstrated in a study of 82 patients with psoriasis, that those randomized to a diet low in calories and fat for 4 weeks had a greater, and statistically significant, reduction in levels of total cholesterol, triglycerides, and low-density lipoproteins, and experienced an improvement in their psoriasis relative to a control group of patients randomized to a standard hospital diet. The greatest contribution to this research was made by Setty et al.,91 who published data on 78 626 women (of whom 892 reported having psoriasis) in a nurses’ health study. Their data indicated that increased adiposity and weight gain are risk factors for the development of psoriasis. Furthermore, they found a linear correlation between BMI and the risk of incident psoriasis, with an increased relative risk of 2.69 for patients with a BMI of 35 or more compared with those with a BMI between 21 and 23.
A number of isolated cases and small series also show a similar association between psoriasis and obesity. Porres92 published the case of a woman aged 44 years with extensive psoriasis who was able to discontinue psoriasis treatment after she lost 54 kg following a jejunoileal bypass. Complete remission of severe generalized psoriasis without medication 1 year after a gastric bypass is reported in 2 other cases in the literature.93,94
Pathogenic Link Between Psoriasis and ObesityAs we have seen above, there appears to be clear epidemiologic evidence for the relationship between psoriasis and obesity, although we now need to establish whether such a relationship is scientifically coherent and, more importantly, whether there is sufficient pathogenic evidence to support the theory. It would seem reasonable to postulate that the low-grade chronic proinflammatory state present in both these conditions increases the risk of the consequences of this state, including a higher likelihood of developing diabetes or metabolic syndrome, and an increase in cardiovascular risk.53 At this point, we can also ask to what extent there may exist a common genetic predisposition for developing both psoriasis and obesity in certain individuals or through what mechanisms the presence of psoriasis might favor obesity and vice versa.
It has been shown that HLA-Cw6, the most important locus of genetic susceptibility for psoriasis, is also associated with obesity. The authors of 1 study found that obese patients carrying HLA-Cw6 were 35 times more likely to develop psoriasis than HLA-Cw6-negative patients of normal weight.95
The fact that obesity and psoriasis can occur together may lead to an interaction between the 2 conditions, in both of which adipokines may play a modulatory and sometimes even a shared role.
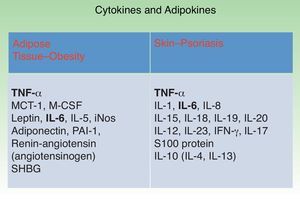
Figure 3 shows the main cytokines and adipokines secreted by visceral AT in obesity and those secreted by the skin in psoriasis. Both tissues secrete TNF-α and IL-6, revealing the similarity in—and probably the synergy between—the inflammatory states associated with these conditions.10,11
Impact of Obesity on the Treatment of PsoriasisThe treatment of obese patients with psoriasis poses numerous difficulties. On the one hand, obesity has been associated with a decreased response to systemic and biologic therapies. This may be due to pharmacokinetic factors and affect more particularly the drugs administered in fixed doses than those in which dose is adjusted to the patient's weight. On the other hand, obesity is associated with conditions such as metabolic syndrome and hepatic steatosis, which can increase the risk of adverse effects to conventional systemic treatment for psoriasis.96
In addition to the potential decrease in the effectiveness of treatment and the greater risk of adverse effects, obesity also substantially increases the cost of treatment with drugs prescribed in weight-adjusted doses.
Effect of Obesity on Conventional Systemic Drug TreatmentTopical Treatments and PhototherapyThere is only anecdotal evidence for the use of topical treatments, including corticosteroids, calcipotriol, tazarotene, and the combination of calcipotriol and betamethasone dipropionate in obese patients with psoriasis.83
Since the dose of psoralen is weight-adjusted, the effectiveness of photochemotherapy with psoralen plus UV-A (PUVA) is unaffected by obesity.83
MethotrexateObesity can complicate the treatment of psoriasis with conventional systemic agents such as methotrexate, since conditions associated with obesity, such as nonalcoholic steatohepatitis, contribute to the hepatotoxicity of methotrexate and are relative contraindications to such treatment.97,98
In patients with psoriasis treated with methotrexate, studies have shown that obesity is an even greater risk factor for hepatotoxicity than alcohol, a history of viral hepatitis, or the cumulative dose of methotrexate, especially when combined with other risk factors, such as diabetes mellitus. As steatosis—a condition that is highly prevalent in obese and/or diabetic patients—probably contributes to this risk, liver toxicity should be monitored more closely in these patients.99–101
The increased risk of liver cirrhosis in obese patients on methotrexate should be viewed as a cause for concern. Although a liver biopsy is traditionally recommended when a patient reaches a cumulative dose of 1.5 g, many authors suggest that in obese patients on methotrexate the liver should be biopsied at a lower cumulative dose.102–105 In a study of patients treated with methotrexate, Berends et al.99 found that 4 out of 38 obese patients and 2 out of 9 patients with diabetes had a Roenigk grade iii or iv liver biopsy result, while none of the 34 patients without risk factors had any liver injury. Weinstein et al.102 found concomitant obesity and diabetes to be significantly associated with fibrosis (mean grade, 3.3) and cirrhosis (mean grade, 1.7) in patients before taking methotrexate. Paradoxically, however, after methotrexate therapy, these conditions were not associated with either fibrosis (1.8) or cirrhosis (2.0) compared with the rest of the study participants. In general, the results of all these studies would appear to indicate that the use of methotrexate is not contraindicated in obese patients with psoriasis, but they do highlight the need for a relatively early follow-up by means of a liver biopsy.
In a study of 500 patients, obesity did not influence tolerance or response to treatment with topical corticosteroids, methotrexate, or PUVA.86 However, loss of response over time was more likely in obese patients treated with methotrexate.
On the other hand, due to its effect on chronic inflammation, treatment with methotrexate is associated with a decreased risk of cardiovascular disease and myocardial infarction, especially when administered at low doses and in combination with folic acid.106
Ciclosporin AThe pharmacokinetics of ciclosporin are influenced by obesity because this highly lipophilic drug is distributed in AT and binds to lipoproteins, which are generally found in larger quantities in obese patients. However, in some studies in renal or bone marrow transplant recipients, obesity was not found to alter the clearance, volume of distribution, or blood levels of ciclosporin.107,108 However, various confounding factors may exist in transplant recipients that may distort the effect of obesity on ciclosporin pharmacokinetics, such as the patient's condition, the use of corticosteroids, and alterations in blood proteins and lipids.
Since these factors are less likely to occur in patients with psoriasis, researchers studied the relationship between obesity and serum concentrations of ciclosporin in 16 patients with psoriasis, and found a strong positive correlation between the standardized trough concentration (concentration in ng/mL divided by the daily dose in mg/kg) and the obesity index used (% obese = 100 [(weight (kg)/22 height (m)2)–1]). Thus, for a given dose of ciclosporin, obese patients are more likely than normal-weight patients to have increased serum levels and, consequently, nephrotoxicity. These results were independent of hematocrit and plasma lipids values, an indication that obesity has an independent effect on ciclosporin levels in patients with psoriasis.109
Other studies confirm that obesity is a risk factor for adverse events in patients treated with ciclosporin, especially in the presence of other factors, such as advanced age, hypertension, and concomitant use of nephrotoxic drugs. In such cases, therefore, the dose of ciclosporin should be adapted to the ideal weight instead of the patient's actual weight in order to reduce the risk of nephrotoxicity.110
Thaçi et al.111 studied the safety and effectiveness of ciclosporin administered over 12 weeks in a new regimen that did not take body weight into account (100-300 mg/d) and compared it with the conventional weight-adjusted dosage regimen (1.25-5 mg/kg/d). The increase in creatinine levels was higher in the group of patients who received the weight-adjusted dose than in the group on the new, weight-independent regimen. This finding could serve as a useful guide in obese patients with a higher risk of liver toxicity if ciclosporin is administered according to real rather than ideal weight.
Thus, close monitoring of serum drug levels, hypertension, liver toxicity, diabetes, and dyslipidemia is essential in obese patients with psoriasis treated with ciclosporin A.
In addition to increasing the risk of toxicity in patients treated with ciclosporin, obesity also appears to influence the effectiveness of such treatment. Gisondi et al.112 showed that moderate weight loss (5%-10% of body weight) increased response to treatment with low doses of ciclosporin in obese patients with moderate to severe psoriasis. The efficacy of ciclosporin (2.5 mg/kg/d) in combination with a low-calorie diet was compared to that of a control group who received ciclosporin alone in 61 obese patients (BMI > 30 kg/m2) with moderate to severe psoriasis. At week 24, the mean (SD) reduction in body weight was 7% (3.5%) in the first group and 0.2% (0.9%) in the control group. In total, 66.7% and 86.7% of the patients treated with ciclosporin and a calorie-controlled diet achieved a reduction in Psoriasis Area and Severity Index of 75% (PASI 75) and 50% (PASI 50), respectively, compared to 29% and 48.3% of patients treated with ciclosporin alone (P < .001).
AcitretinHypercholesterolemia with decreased high-density lipoproteins is a common side effect in patients with psoriasis treated with acitretin, especially those with diabetes, obesity, alcohol dependency, or hypertriglyceridemia.113
Corbetta et al.114 studied the effects of acitretin on lipid and glucose metabolism after 1 and 3 months of treatment in 10 patients with psoriasis. The changes observed in glucose tolerance and lipid metabolism were transient and not associated with changes in BMI or in the levels of TNF-α or the hormones involved in obesity (resistin and adiponectin).
Effect of Obesity on Treatment with BiologicsAlthough the results of many studies cannot be compared owing to differences in the methods used to quantify body weight, it is possible that biologic drugs, particularly those in which dose is not weight-adjusted, may be less effective in overweight patients.115,116
AT, and especially abdominal AT, is an endocrine organ that secretes fatty acids, proinflammatory cytokines (TNF-α, IL-1, IL-6, IL-10), hormones (leptin, adiponectin), and prothrombotic factors. Since the role of TNF inhibitors is to eliminate excess TNF-α from the circulation and from psoriatic plaques, and since TNF production is increased in obese patients, these drugs may in fact be less effective in obese patients.106,117
In an Italian study, Naldi et al118 analyzed data from the Psocare program, which collects data from a number of clinics relating to patients with psoriasis who are starting a new treatment (conventional or systemic), and found that BMI was a predictor of treatment response. The proportion of patients who achieved a PASI 75 response decreased with increasing BMI. At 8 weeks, 41.7% of the patients with a BMI under 20 achieved PASI 75 compared to 29.1% of those with a BMI of 30 or higher. The corresponding figures at 16 weeks were 59% for the group with the lowest BMI and 42.2% for those with the highest. Obese patients were less likely than normal-weight patients to show improvement at 8 and 16 weeks, independently of other variables, such as sex, severity of psoriasis, and prior treatment. The OR for achieving a PASI 75 response in the obese patients relative to normal-weight patients was 0.73 (95% CI, 0.58-0.93) at 8 weeks and 0.62 (95% CI, 0.49-0.79) at 16 weeks.
In the following section the evidence on the impact of obesity on treatment outcomes is evaluated in detail for the different biologic agents.
InfliximabThe weight-adjusted dosing of infliximab makes it possible to obtain similar results in obese and nonobese patients. In an analysis of a subgroup of 1462 patients treated with infliximab in 3 clinical trials, Reich et al.119 found a similar response (PASI 75) after 10 weeks of treatment in overweight or obese patients and in normal-weight patients (BMI < 25 kg/m2). The PASI 75 response at 10 weeks was comparable in overweight patients (78.3%), obese patients (74.4%), and normal-weight patients (77.5%).
In another study of 53 patients with moderate to severe psoriasis treated with infliximab (5 mg/kg at weeks 0, 2, 6, and every 8 weeks thereafter), obesity was associated with a delay in response and lower efficacy.120
EtanerceptStrober et al.121 studied the effect of various demographic factors on the efficacy of etanercept. Patients were randomized to receive etanercept 50 mg or placebo for 12 weeks; this was followed by a 36-week open-label phase with etanercept 50 mg. Seventy percent of the patients were overweight and 10% were morbidly obese. Response to etanercept was better in the patients with a BMI within the normal range than in the overweight or obese patients. In the group of morbidly obese patients (BMI ≥ 40), the response at 12 weeks was as follows: PASI 90 in 15%, PASI 75 in 25%, PASI 50 in 32%, and a response less than PASI 50 in 27% of patients. Among normal-weight patients, the percentages were 41%, 33%, 17% and 9%, respectively.
In an integrated analysis of 1187 patients treated with etanercept (either 50 or 100 mg/wk) or placebo, Gordon et al.122 observed better response in patients with lower weight and in those receiving etanercept 100 mg/wk. At week 12, 41% of the patients who weighed under 89.36 kg and received etanercept 50 mg/wk achieved a PASI 75 response, as compared with 25% of the heavier patients on the same dose. In the group receiving 50 mg twice weekly, 53% of the patients weighing under 89.36 kg and 43% of those weighing more achieved PASI 7.
However, in a study of 100 patients with psoriasis treated with etanercept 100 mg/wk for 12 weeks, followed by 50 mg/wk, no relationship was found between treatment efficacy and BMI at 12 or 24 weeks.123
Similarly, body weight was not observed to influence response to etanercept in another study of 50 patients randomized to receive etanercept 25 or 50 mg twice weekly.124 Although almost all of the patients who did not achieve PASI 50 in that study had a high BMI (25.8-30 kg/m2), there were also a considerable number of overweight or obese patients in the group that achieved a PASI 75 response.
AdalimumabIn the subanalyses of the REVEAL, BELIEVE, and CHAMPION trials, response to treatment also decreased with increasing body weight, although the reduction was often not significant.125–128
In the REVEAL trial, the factors that most influenced treatment response were type of treatment, weight, and age. In the patients treated with adalimumab, 74.1% of patients who weighed under 100 kg achieved a PASI 75 response at week 16, as compared to 63.8% of the patients weighing more than 100 kg. When BMI was taken into account, the percentage of responders was 79.2%, 75.5%, and 65.1% for patients with normal weight, overweight, and obesity, respectively.129,130
In a subanalysis of data from the BELIEVE study, the efficacy of adalimumab, alone or in combination with calcipotriol and betamethasone was analyzed according to the characteristics of psoriasis and patient-related factors. The percentage of patients who achieved PASI 75 at 16 weeks was lower in the group weighing 95 kg or more.131
One of the objectives of the CHAMPION trial was to determine the effect of patient-related factors and the characteristics of psoriasis on the efficacy of adalimumab, compared with that of methotrexate and placebo. Response to treatment, assessed in terms of the number of patients who achieved a PASI 75 response at week 16, was analyzed according to patient weight divided into quartiles (≤ 68 kg, > 68 and ≤ 82 kg, > 82 and ≤ 92 kg, > 92 kg). The response (≥ PASI 75) by quartile was 85%, 86%, 86% and 60%, respectively; there were no significant differences in the efficacy of adalimumab associated with weight.128
In patients with rheumatoid arthritis on adalimumab, an increase in the serum clearance of the drug was found in the heavier patients, an effect that could lead to a reduced efficacy.132
UstekinumabIn the PHOENIX 1 and PHOENIX 2 trials researchers found a correlation between body weight, serum ustekinumab concentrations, and efficacy; no differences in safety were observed.133 In these 2 phase III trials, patients were randomized to receive 45 mg or 90 mg of ustekinumab every 12 weeks or placebo with crossover to ustekinumab at week 12. Serum ustekinumab concentrations and efficacy were assessed by 10-kg increments of body weight at week 28. Among the patients who weighed more than 100 kg, response (PASI 75) was 20% higher in the group receiving 90 mg of ustekinumab than in the group receiving 45 mg (74.2% vs 54.6%, P < .0001). However, among patients who weighed under 100 kg, no significant differences in response to treatment were observed for the 2 dose levels (80.8% in the 90-mg group and 76.9% in the 45-mg group; P = .1823).
These differences in efficacy were also observed when response was measured as the percentage of patients achieving PASI 90 or a Physician's Global Assessment score of 0 or minimal disease.
Serum ustekinumab concentrations were also affected by weight, with lower concentrations in heavier patients. In parallel with clinical efficacy, serum concentrations in patients who weighed 100 kg or less and received a 45-mg dose were similar to those found in patients who weighed over 100 kg treated with ustekinumab 90 mg. These findings will presumably be reflected in the Summary of Product Characteristics, and a dose of 90 mg will be recommended for patients weighing more than 100 kg and a dose of 45 mg for patients below this weight.
The incidence and type of adverse events were similar and were independent of the dose administered and the patient's weight. The percentage of autoantibodies tended to be higher in the heavier patients treated with the 45-mg dose, suggesting that their development may be related to the presence of drug concentrations below detectable limits.133
The ACCEPT trial, which compared the efficacy and safety of ustekinumab (45 or 90 mg) with that of etanercept, reported similar findings, with a better response (PASI 75 at 12 weeks) in patients weighing less than 100 kg and in those treated with ustekinumab.129
Effect of Biologic Treatments on Body WeightSeveral studies have shown that the treatment of moderate to severe psoriasis can lead to a moderate increase in the patient's weight, a difference that becomes significant after 12 weeks of treatment.134 Based on these results, it is thought that TNF-α inhibition, which normally induces the production135 and release136 of leptin, may lead to increased appetite, a hypothesis that would explain the weight gain observed in this setting. However, the proven effectiveness of the biologic treatment in these patients clearly tips the balance in the risk/benefit assessment.
Role of Obesity in the Choice of Biologic TherapySeveral studies have shown the importance of adjusting the dose of biologic drugs according to weight to ensure the drug levels associated with optimal effectiveness and the need to avoid excessive doses in patients with lower weight.
Although there is no protocol for switching from one biologic agent to another, some authors consider that body weight is a factor that should considered in the choice of treatment. Thus, in heavier patients it would be reasonable to consider as a first-line choice those biological drugs in which dose is weight-adjusted, such as infliximab and ustekinumab. However, the increased cost of treatment should also be considered. In the clinical trials of etanercept and adalimumab, some patients who did not respond to conventional doses of the drug responded when the dose was doubled, but this strategy would represent a significant increase in cost and drug exposure.130,131 Practical recommendations have recently been published on the diagnosis, implications, and therapeutic management of obese patients with psoriasis.137,138
Conclusions Regarding TreatmentThe treatment of obese patients with psoriasis involves problems, such as the increased risk of adverse effects in the case of conventional systemic drugs, and the reduced efficacy and/or higher cost of treatment in the case of biologic agents.
One of the aims of the management of obese patients with psoriasis should be to achieve a reduction in the patient's weight and, consequently, in the associated inflammation. This measure will decrease the risk of toxicity and enhance effectiveness and tolerance, particularly in the case of drugs administered at fixed doses.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no private patient data are disclosed in this article
Right to privacy and informed consentThe authors declare that no private patient data are disclosed in this article
FundingMSD supported the writing of this article but did not contribute in any way to its content, which has been freely chosen and written by the authors
Conflicts of InterestsDr. José Manuel Carrascosa has participated in studies and presentations sponsored by Pfizer, MSD, Johnson, and Abbott.
Dr. Vicenç Rocamora has participated in studies and presentations sponsored by Pfizer, MSD, Johnson, and Abbott.
Dr. José C Moreno-Giménez has participated in research projects and presentations sponsored by the following pharmaceutical companies: Janssen, MSD, Abbott, Leo-Pharma, Galderma, and Pfizer.
The remaining authors declare no conflicts of interest.
Please cite this article as: Carrascosa JM, Rocamora V, Fernandez-Torres RM, Jimenez-Puya R, Moreno JC, Coll-Puigserver N, et al. Obesidad y psoriasis: naturaleza inflamatoria de la obesidad, relación entre psoriasis y obesidad e implicaciones terapéuticas. Actas Dermosifiliogr. 2014;105:31–44.