Drug-induced lupus erythematosus (DILE) refers to a condition whose clinical, histological, and immunological features are similar to those seen in idiopathic lupus erythematosus but that occurs when certain drugs are taken and resolves after their withdrawal. Over 90 drugs have been linked to DILE to date and the list is growing. Like idiopathic lupus erythematosus, DILE has systemic, subacute cutaneous, and chronic cutaneous forms. A correct diagnosis is very important, as this condition usually resolves after withdrawal of the offending drug.
El término lupus eritematoso inducido por fármacos (LEIF) hace referencia a una entidad caracterizada por la aparición de manifestaciones clínicas, histopatológicas e inmunológicas similares a aquellas que aparecen en el lupus eritematoso idiopático, pero que cronológicamente coinciden con la toma de ciertos fármacos y que se resuelven tras la retirada de los mismos. Más de 90 fármacos se han asociado con la aparición de LEIF. Esta lista de fármacos implicados sigue aumentando. Al igual que el lupus eritematoso idiopático, el LEIF se puede subclasificar en lupus eritematoso sistémico inducido por fármacos, lupus eritematoso cutáneo subagudo inducido por fármacos y lupus eritematoso cutáneo crónico inducido por fármacos. Reconocer estas entidades es de gran interés, ya que este cuadro suele revertir tras la retirada del fármaco implicado.
Autoimmune disorders arise from changes in the regulation of one or more components of immune response and involve multiple genetic, epigenetic, and environmental factors that play important roles in triggering and maintaining the process.
Drug-induced autoimmune syndromes have been recognized for some time. The classic example is drug-induced lupus erythematosus (DILE).
Systemic lupus erythematosus (SLE) is an autoimmune disease of unknown origin, but many drugs have been seen to induce lupus-like signs and symptoms. The resulting condition has been referred to variously as DILE, lupus-like syndrome, medicine-induced lupus erythematosus, or drug-related lupus. The condition develops in patients with no history of autoimmune disease and accounts for 10% to 15% of all SLE cases.2
The incidence of drug-induced autoimmunity has risen considerably in the last 10 years, and over 90 drugs have been implicated to date. The rapid rise in the number of culprit medications may be attributable to the introduction and use of newly developed drugs in the last 10 years. For example, biologic agents created to block specific phases of the immune response can trigger significant changes in the system. That drugs such as hydralazine, procainamide, isoniazid, quinidine, and chlorpromazine can cause DILE is unquestioned. The highest risk is associated with just 2 drugs: procainamide,3 which has been linked to DILE in around 20% of treated patients, and hydralazine,4 which has caused the disorder in 5% to 8% of patients treated at least a year according to standard dosing regimens. On the basis of the low number of cases linked to other drugs, it can be inferred that they seem to involve less risk.
ConceptAlthough diagnostic criteria are not well established for systemic DILE, it is widely defined as a disorder that resembles SLE but develops when a drug is being taken continuously for at least a month and that disappears when treatment is discontinued. Like lupus, DILE generally causes fever, musculoskeletal pain, and serositis. This clinical picture is usually accompanied by characteristic laboratory findings, specifically serum positivity for antinuclear antibodies (ANA) and antihistone antibodies (AHA). Idiopathic SLE can be difficult to distinguish from systemic DILE given that these 2 entities have similar clinical, serologic and histologic findings. The presence of certain serum markers, and especially resolution of symptoms when treatment with the culprit drug is discontinued, assist in establishing the correct diagnosis.
EpidemiologyThe incidence of DILE in the United States has been estimated to be somewhere between 15 000 and 30 000 new cases every year, meaning that the number of patients with this form may be about 10% to 15% of the number with idiopathic SLE.1 Whereas women with idiopathic SLE outnumber men, systemic DILE affects men and women in similar proportions.5 Patients with systemic DILE also tend to be older, as patients of advanced age are often prescribed more medications. The prevalence has also been shown to be 6-fold higher in white patients than in black patients.6 To date, the drug most often associated with systemic DILE is procainamide.
Etiologic and Pathogenic MechanismsThe etiology and pathogenesis of DILE remains to be fully elucidated, but this disorder probably arises from a combination of features specific to the culprit drug itself and others that are patient-related.
DILE differs from drug hypersensitivity reactions in several ways. First, no evidence of drug-specific T cells or antibodies has been found, nor have target autoantigens directly affected by the culprit drug been detected. Second, DILE develops in relation to the cumulative dose taken and it can take months or even years for symptoms to appear. Finally, symptoms generally take a day or 2 to reappear after rechallenge, indicating the absence of a specific hypersensitivity immune response. It is believed that the development of DILE requires a certain degree of genetic susceptibility, given that patients with relatives who have SLE are more likely to develop the drug-induced form. An individual's acetylator status, which is determined genetically, is also known to play a role.7 Slow acetylators are homozygous for the recessive gene that controls expression of the liver enzyme acetyltransferase, which is involved in the metabolism of certain implicated drugs, such as procainamide, isoniazid, and hydralazine. Slow acetylators treated with hydralazine or procainamide have also been reported to develop ANA positivity more quickly than fast acetylators and to progress to symptomatic DILE more often. The genetic susceptibility hypothesis recently gained ground after association with certain major histocompatibility complex (HLA) phenotypes. Certain HLA haplotypes—HLA-DR2, HLA-DR4, and HLA-DR3—as well as a slow acetylator phenotype and an unexpressed or “null” allele for C4 may be linked to the development of autoimmunity.8 It is also thought that certain events must occur in sequence before DILE develops.9 The drug must first undergo biotransformation to a pharmacologically reactive form. The resulting metabolites would have the ability to form stable complexes with self-macromolecules or to directly stimulate lymphocytes, initiating changes that significantly affect the immune system. In this process, activated neutrophils in the bloodstream have been shown to take part in the oxidative metabolism of many drugs implicated in DILE, facilitating the formation of metabolites that trigger autoimmunity10; drugs biotransformed in this way include procainamide, hydralazine, quinidine, phenytoin, sulfone, propylthiouracil, penicillamine, chlorpromazine, isoniazid, and carbamazepine. A strong correlation between a drug's susceptibility to myeloperoxidase-mediated oxidative transformation and the tendency of the drug to induce lupus erythematosus has been demonstrated.10
The formation of reactive metabolites with similar characteristics could explain why chemically and pharmacologically different drugs are able to induce similar clinical manifestations and trigger autoimmunity.
After these biotransformations, autoimmunity can then be triggered by either the drug itself or its metabolites. Various theories to explain this process have been put forth. One hypothesis postulates that drugs can cause DILE by altering T-cell cell DNA methylation,11 which has a primordial role in the regulation and expression of genes and cell differentiation. Drugs like procainamide and hydralazine decrease methylation by inhibiting DNA methyltransferase. Impaired DNA methylation in T cells would change their genetic expression and function. A second theory is that autoreactive B cells are activated by drug-specific T cells. A final hypothesis proposes that instead of stimulating mature T cells directly, the culprit drug subverts de novo acquisition of B-cell tolerance if it is present in the thymus as these cells develop.12,13 T cells are generated in the thymus throughout life, and the injection of a metabolite of procainamide into the thymus has been shown to lead to late formation of anti-([H2A-H2B]-DNA) metabolites.14 Many drugs are also believed to have the ability to interact with nuclear proteins such as histones,15 leading to the formation of molecules that would express new antigenic products, enhancing the production of AHAs. In addition, the molecular structure of certain drugs resembles that of purine bases, favoring a DNA cross-reaction.16 Anti-tumor necrosis factor (TNF) agents appear to cause autoimmune reactions by other mechanisms. According to some hypotheses, anti-TNF antibody binding to the cell surface induces apoptosis,17 causing the release of antinucleosome antibodies and the induction of anti-double-stranded DNA (anti-dsDNA) antibodies. Another theory states that anti-TNF agents would cause a change in the helper T (TH) cell profile. TH2 cytokines are thought to play a role in SLE by activating B cells. Anti-TNF agents block the TNF TH1 cytokine, possibly driving a shift in the immune system toward a TH2 profile with production of antibodies and the development of lupus-like symptoms. The last hypothesis postulates that bacterial infections promoted by anti-TNF therapy could provide the stimulus for polyclonal B-cell activation and consequent release of antibodies.18
In discussing the etiology and pathogenesis of drug-induced subacute cutaneous lupus erythematosus (DISCLE), Sontheimer and colleagues19 recently pointed out that most of the drugs implicated in this disorder also cause photosensitivity or a lichenoid photosensitive reaction. The authors noted that this would explain a photoinduced isomorphic response in immunogenically predisposed individuals. Another possibility along the same line is that cutaneous inflammation from a photosensitive drug can change the innate immune response, leading to a local increase in type 1 interferon (IFN) production by dendritic cells. The result of dysregulation is an imbalance in the cascade (e.g., the production of CXCL9, CXCL10, and CXCL11 and consequent CXCR3-mediated recruitment of cytotoxic T cells); this process occurs in most conditions involving interface dermatitis, including SLE.
Clinical ManifestationsThe clinical spectrum of DILE ranges from circumscribed cutaneous signs to systemic involvement, which is usually mild.2 The onset of DILE is usually insidious, and the clinical picture varies widely.
Systemic DILEThe main symptoms in systemic DILE are musculoskeletal (joint and muscle) pain, serositis, and constitutional manifestations such as fever, fatigue, and loss of appetite. These symptoms are usually mild.
Arthritis is usually symmetric, affects small joints (e.g., in the hands), and does not lead to deformation. This clinical pattern is typical of hydralazine-induced cases. The severity of the myalgia that is also very common in patients with systemic DILE bears a relation to the length of time the patient took the culprit drug.20
Classic mucocutaneous signs, such as malar erythema, discoid lesions, hair loss, or oral ulcers are common in systemic DILE, and serositis often develops. The course of pericarditis is usually benign in patients with this form of the disorder, although constrictive pericarditis, cardiac tamponade, and pericardial effusion have been reported.21–23 Pleuritis, pleural effusion, and pulmonary infiltrates are not uncommon when the culprit drug is procainamide.24 Renal or neurologic involvement, on the other hand, is uncommon, distinguishing systemic DILE from idiopathic SLE. When systemic DILE affects the kidneys, hydralazine is most often the trigger.25
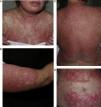
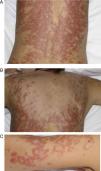
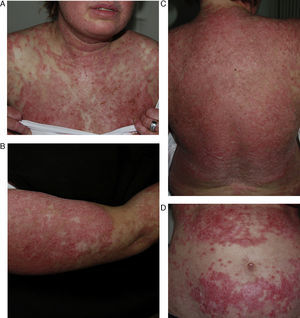
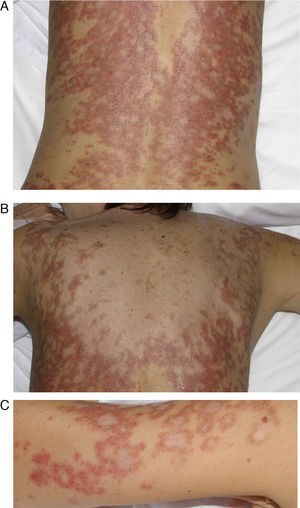
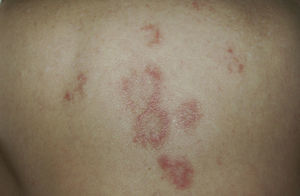
DISCLEThe clinical and laboratory findings are similar in DISCLE and the idiopathic form of subacute cutaneous lupus erythematosus. Both conditions are more prevalent in women, but patients with the drug-induced form are usually older.26 The latency period between starting the drug and the onset of symptoms ranges from 4 to 20 weeks. Cutaneous signs include an eruption of papulosquamous lesions in sun-exposed areas, such as the face, neck and throat, and the outer surface of the arms (Fig. 1); lesions may take an annular form (Figs. 2 and 3) and they resolve within weeks or months without leaving scars.27 DISCLE lesions may also be more widely disseminated, extending to unexposed skin areas such as the lower third of the back.
A 29-year-old woman with a history of epilepsy and carbamazepine-induced subacute lupus erythematosus that first appeared 9 years earlier. A, Note the intense papulosquamous psoriasiform lesions on the chest and face. B, Significant eruption on the upper arm and forearm, with poikilodermal areas. C and D, Large erythematous, scaly plaques covering the back and a large portion of the abdomen. The culprit drug could not be discontinued because epileptic seizures were poorly controlled with alternatives.
A 54-year-old woman with a history of epilepsy and subacute lupus erythematosus after treatment with oxcarbazepine started 2 months earlier. A, B, and C, Scaly, erythematous annular lesions on the lumbar region of the back, shoulder, and arms, respectively. Symptoms resolved 3 months after the antiepileptic drug was discontinued.
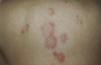
A 79-year-old woman with a history of metastatic ovarian cancer. The back lesions are clinically and histopathologically compatible with subacute lupus erythematosus of 2 months’ duration. The patient reported having taken bisoprolol in recent months, and the lesions resolved after the drug was stopped.
Some authors note that blistering lesions are more common in DISCLE than in the idiopathic form of subacute cutaneous lupus.28 Noncutaneous manifestations, such as arthritis, serositis, and visceral organ involvement are not normally seen in DISCLE.29 Anemia and low white blood cell or platelet counts are very rarely found. Symptoms improve in most patients 8 weeks after withdrawal of the drug and anti-Ro antibodies disappear by 8 months.26
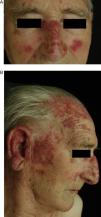
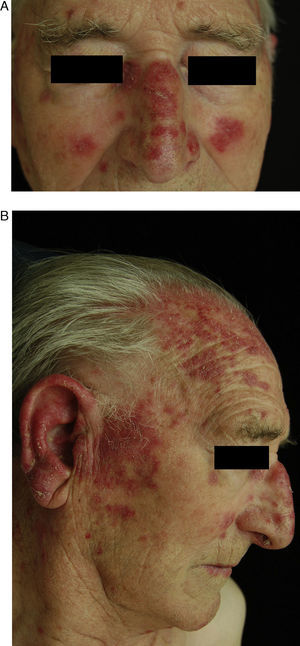
Chronic Cutaneous DILEThe clinical manifestations of chronic cutaneous DILE are the classic discoid lesions in sun-exposed areas like the face, upper trunk, and arms (Fig. 4). As in the idiopathic form of this disease, systemic manifestations are usually absent. The literature indicates that some 8 months pass between initiating treatment with the culprit drug and the onset of symptoms. Clinical manifestations typically resolve about 5 weeks after withdrawal of the drug.30
A and B, An 82-year-old man with scaly, erythematous lesions on the face and arms that appeared 9 months earlier. Histopathology was compatible with chronic discoid lupus. The patient had been treated with hydrochlorothiazide. The lesions resolved several months after treatment was discontinued.
The drugs linked to systemic DILE can be grouped in 3 categories (Table 1).
- 1.
Category 1: Drugs that have been clearly demonstrated to cause systemic DILE. Convincing evidence has been found for the following drugs: hydralazine, procainamide, isoniazid, methyldopa, quinidine, and chlorpromazine.
- 2.
Category 2: Drugs that may cause this condition. Anticonvulsant and antithyroid agents, D-penicillamine, sulfasalazine, β-blockers, and thiazide diuretics fall into this category.
- 3.
Category 3: Drugs for which at least 1 case of systemic DILE has been reported in the literature. Examples are minocycline and some other tetracyclines, valproic acid, IFN-α, interleukin (IL) 2, clobazam, lamotrigine, infliximab, angiotensin converting enzyme inhibitors, ticlopidine, amiodarone, lipid lowering agents, gold salts, penicillin, streptomycin, phenylbutazone, estrogens and oral contraceptives, para-aminosalicylic acid, and reserpine.
Drugs Reported to Have Induced Systemic Lupus Erythematosus.
| Drug Group | High Risk | Moderate Risk | Low Risk | Very Low Risk |
| Antiarrhythmia agents | Procainamide (15%–20%) | Quinidine (<1%) | Disopyramide, propafenone | |
| Antihypertensives | Hydralazine (5%–8%) | Methyldopa, captopril, acebutolol | Clonidine, enalapril, labetalol, minoxidil, pindolol, prazosin, atenolol, timolol | |
| Antipsychotic agents | Chlorpromazine | Chlorprothixene, lithium carbonate, phenelzine, perphenazine | ||
| Antibiotics | Isoniazid | Minocycline | Nitrofurantoin | |
| Anticonvulsants | Carbamazepine | Ethosuximide, phenytoin, primidone, trimethadione | ||
| Antithyroid agents | Propylthiouracil | |||
| Anti-inflammatory drugs | Sulfasalazine | D-penicillamine, sulfonamide | Phenylbutazone | |
| Diuretics | 5-aminosalicylic acid | Chlorthalidone, hydrochlorothiazide | ||
| Lipid lowering agents | Atorvastatin, fluvastatin, lovastatin, pravastatin, simvastatin | |||
| Biologic agents | Etanercept, infliximab, adalimumab, IFN-α, interleukin 2 | |||
| Neuroleptic agents | Levodopa | |||
| Adrenal steroid inhibitors | Aminoglutethimide |
Abbreviation: IFN, interferon.
A peripheral vasodilator used to treat arterial hypertension since the 1950s, hydralazine is currently being replaced by newer drugs with more acceptable safety profiles. The annual incidence of systemic DILE in patients treated with standard doses of hydralazine is between 5% and 8%,31 and this drug—alongside procainamide—is associated with the greatest risk. Hydralazine is absorbed in the gastrointestinal tract and metabolized in the liver by N-acetyltransferase in a genetically determined process. Two acetylator types have been described, according to whether this process takes place slowly or rapidly. In slow hydralazine acetylators, ANA positivity is usually detected at lower doses of the drug within a shorter period of time. Acetylation rate is not the only factor that affects the probability of developing DILE, however. Drug dose and the patient's HLA profile are also relevant. Many patients develop DILE at hydralazine doses as low as 100mg,32 and the condition has been associated with the presence of HLA-DR4, supporting a genetic component.33 Although up to half of patients who take hydralazine at doses of 200mg/d show ANA positivity,2 only 10% of hydralazine-treated patients overall have systemic DILE. There appears to be a correlation between the development of autoimmunity and hydralazine dose, an association not seen with other drugs. Some authors have found that systemic DILE developed in 5.4% of patients taking hydralazine at a dosage of 100mg/d, whereas the disease presented in 10.4% taking 200mg/d. Patients with systemic DILE triggered by hydralazine often suffer joint disease, such as arthritis, which is found in 63% of them. Although renal involvement is generally uncommon, hydralazine is the drug implicated most often when this complication does occur.25 Very few instances of lung involvement—pleuritis, pulmonary vascular disease or pneumonitis—have been described in hydralazine-induced lupus.34 Cutaneous involvement is more prevalent in DILE induced by hydralazine than in those linked to procainamide, although the presentation is not the typical one of malar erythema.35
Finally, serum ANA and AHA positivity is common (in 95% of patients) along with anti-dsDNA negativity (in 5%) and a normal complement profile.35
ProcainamideAn antiarrhythmic agent used since the 1950s, procainamide is one of the most common causes of drug-induced autoimmunity. Nearly 90% of procainamide-treated patients have detectable ANA levels, although only 30% develop symptomatic DILE.36 Time from start of treatment to onset of symptoms ranges from 3 months to 2 years. As mentioned above, just as cutaneous and renal involvement are very uncommon in systemic DILE due to hydralazine, pleural and pericardial involvement are especially frequent in cases induced by procainamide.35 Immunologic abnormalities are similar in both procainamide- and hydralazine-induced cases, but a high percentage of patients who have taken procainamide have AHA to the (H2A-H2B)-DNA complex.37
IsoniazidUp to 25% of patients taking this antituberculosis drug have detectable ANA titers, but only 1% of these patients develop systemic DILE,2 which requires taking dosages of 300 to 900mg/d for 4 weeks to 14 months. As is the case for patients on hydralazine therapy, slow acetylators are at higher risk. The most frequent clinical manifestations are joint pain or arthritis and anemia. Fever and pleuritis occur in half of patients who develop systemic DILE, and pericarditis develops in 30%.
A finding of anti-immunoglobulin (Ig) G antibodies to the (H2A-H2B)-DNA complex seems to be specific to isoniazid-induced systemic lupus.
MethyldopaAn antihypertensive drug, methyldopa has been prescribed widely even though it has many adverse effects. ANA positivity is detected in 15% of patients on methyldopa, and 1% of them develop systemic DILE.2,38
ChlorpromazineA widely used antipsychotic agent, chlorpromazine is associated with ANA positivity in about 50% of patients treated at dosages of 400mg/d; however, systemic DILE develops in fewer than 5%.39
The most common clinical signs resemble those of idiopathic SLE: fever, joint pain, and skin eruptions. Severe cardiac symptoms have been reported.
QuinidineA drug used to treat cardiac arrhythmia, quinidine has been linked to ANA-negative systemic DILE. This drug is considered to confer intermediate risk.40 Quinidine-induced lupus appears to be associated with lower complement levels, an unusual observation in systemic DILE caused by other drugs.
Category 2SulfasalazineAlso known as salazopyrin, this compound comprised of 5-aminosalicylic acid and sulfapyridine is used in a large number of rheumatic diseases.41–43 Although systemic DILE is infrequent, cases of AHA positivity and anti-dsDNA negativity have been described.
AnticonvulsantsAntiepileptic drugs that have been linked to systemic DILE, with or without detectable ANA, include carbamazepine, diphenylhydantoin, and ethosuximide.
Cases of carbamazepine-induced lupus are rare, with an incidence of less than 0.001% of treated cases.44
Antithyroid agentsPropylthiouracil, thiouracil, and methylthiouracil are widely used to treat hyperthyroidism. DILE induced by these drugs is associated with detection of ANA, anti-dsDNA, antineutrophil cytoplasm antibodies; leukopenia and cutaneous purpura are also reported in these cases.45
D-penicillamineA copper chelating agent used to treat Wilson disease, scleroderma, and rheumatoid arthritis, D-penicillamine has been linked to systemic DILE only rarely.46 Patients on this drug can be asymptomatic but show ANA positivity and low complement levels. Alternatively, serious disease may develop with joint pain or arthritis, serositis, cutaneous eruption, general malaise, and weight loss.
Thiazide diureticsAntihypertensive drugs with a fairly good safety profile, thiazide diuretics have been linked to DISCLE with positive ANA and anti-dsDNA positivity and AHA negativity.43,47
β-BlockersPropranolol and atenolol are among these widely prescribed drugs to treat hypertension or prevent angina or cardiac arrhythmia. Cases of ANA-positive, AHA-negative systemic DILE have been described in association with β-blockers.48
Category 3MinocyclineA semisynthetic tetracycline derivative, minocycline is used to treat acne and other conditions. Although systemic DILE due to minocycline is relatively rare, patients who had taken over 50 000mg of minocycline for acne in a recent controlled trial proved to be at greater risk of developing this autoimmune disorder.49 Women are at higher risk than men, and no link between autoimmunity and other tetracyclines has been found.50 Minocycline-triggered systemic DILE involves classic symptoms, such as joint pain and arthritis, but also unusual skin manifestations (Raynaud phenomenon, polyarteritis nodosa, erythema nodosum) and liver involvement; AHA positivity is seen in some cases.51 A small percentage of patients develop oral ulcers, livedo reticularis, rash, alopecia, and vasculitis; this condition is therefore difficult to distinguish from idiopathic SLE.52 Neurologic, pulmonary, and blood manifestations are rare. Positivity for ANA, anti-smooth muscle and anti-phospholipid antibodies, and anti-DNA are routine findings.52 The clinical picture resolves on discontinuation of the drug, although complete remission can sometimes take up to 2 years.
Valproic acidAn antiepileptic drug, valproic acid can cause systemic DILE characterized by fever, joint pain, low white blood cell or platelet counts, central nervous system involvement, AHA and anti-dsDNA positivity, and low complement levels.
IFN-αBiologics, such as IFN-α and β, are used to treat hepatitis C virus infection, to alter immune system functions, and to induce various autoimmune phenomena.53 A few cases of DILE induced by IFN-α have been reported.54,55
Cases of systemic DILE, induced by this drug show a high rate of mucocutaneous and renal involvement as well as anti-dsDNA positivity in half of patients affected; these observations help distinguish cases caused by this drug from others.
IL-2The IL-2 cytokine produced by TH cells is prescribed as an immunotherapeutic agent for melanoma and renal carcinoma. Cases of systemic DILE have been reported.56
ClobazamThis benzodiazepine, which is used to treat epilepsy, has been reported to have caused systemic DILE.57
LamotrigineThis antiepileptic factor was recently implicated in a case of systemic DILE.58
Anti- TNF agentsEver since anti-TNF agents entered the market, they have been widely prescribed to treat several highly prevalent diseases such as rheumatoid arthritis, psoriasis, spondyloarthropathies, and inflammatory bowel disease. That patients taking these drugs have abnormal antibody profiles is well known, but systemic DILE develops only rarely.59 Clinical trials and postmarketing studies have demonstrated antibodies in nearly two-thirds of treated patients, but DILE develops in fewer than 1%.60 Recent years have seen multiple case reports of systemic DILE in patients on anti-TNF agents, however. The varied clinical picture includes arthritis or joint pain, rash, serositis, and low counts of certain blood cell types. Systemic DILE in patients on anti-TNF therapy differs from the classic presentation in several ways. First, these drugs are associated with a higher incidence of skin manifestations (in 72% of cases17). Second, although visceral organs are seldom involved in classic systemic DILE, this finding is more common in cases triggered by anti-TNF therapy, where kidney involvement in particular is not unusual.61–63 Third, whereas high levels of anti-extractable nuclear antigen and anti-dsDNA antibodies, along with low complement levels, are detected in up to half of cases linked to anti-TNF,17 those findings are rare in classic systemic DILE.17 In contrast, anti-AHA positivity is found more often in the classic form. The anti-TNF agents linked most often to systemic DILE are etanercept, infliximab, and, much less frequently, adalimumab. A total of 92 cases of systemic DILE (40 due to infliximab, 37 to etanercept, and 15 to adalimumab) were found by the authors of an analysis published in 2007.64 Leflunomide, a drug that has been effective against SLE, can itself induce lupus symptoms.65–67 It is unknown whether a patient who has developed systemic DILE on taking an anti-TNF agent would be predisposed to such a response on taking another drug in this class.
DISCLEThe drugs that usually trigger the subacute cutaneous form of lupus are antihypertensives such as thiazides,68,69 angiotensin converting enzyme inhibitors,70 β-blockers,71 and calcium channel blockers (Table 2). DISCLE has recently been described in patients taking terbinafine,72–74 bupropion,75 antineoplastic agents,76–78 leflunomide,65,66 efalizumab,79 proton pump inhibitors,80 tamoxifen,81 neuroleptics (phenytoin,82 oxcarbazepine83), benzodiazepines (tetrazepam, lormetazepam),84 procainamide,84 and etanercept.85
Drugs Possibly Implicated in DISCLE.
| Drug Class | High Risk (>5%) | Low Risk (<5%) | |
| Antifungal agents | Griseofulvin, terbinafine | ||
| Antihypertensives | Calcium channel blockers: diltiazem, verapamil, nifedipine, nitrendipine | Angiotensin converting enzyme inhibitors: cilazapril, captopril | |
| β-blockers: oxprenolol, acebutolol | |||
| Diuretics: hydrochlorothiazide, spironolactone | |||
| Chemotherapeutic agents | Docetaxel | 5-Fluorouracil, capecitabine | |
| Antacids | Omeprazole lansoprazole, ranitidine | ||
| Antiepileptics | Phenytoin, oxcarbazepine | ||
| Antimalarial drugs | Hydroxychloroquine | ||
| Immunomodulators | Etanercept, infliximab, efalizumab, IFN-α, leflunomide | ||
| Lipid lowering agents | Pravastatin, simvastatin | ||
| Anti-inflammatory drugs | Naproxen, piroxicam | ||
| Antidepressants | Bupropion | ||
| Antidiabetic drugs | Sulfonylurea (glyburide) | ||
| Antiarrhythmia agents | Procainamide | ||
| Benzodiazepines | Tetrazepam, lormetazepam | ||
| Platelet aggregation inhibitors | Ticlopidine | ||
| Estrogen receptor antagonists | Tamoxifen | ||
| Miscellaneous | D-penicillamine, insecticides | ||
Abbreviation: IFN, interferon.
The chronic cutaneous form of lupus, which is very rare, has usually been caused by fluorouracil (especially tegafur and tegafur-uracil) or one of its more modern derivatives, such as capecitabine.30,86 Nonsteroidal anti-inflammatory drugs and thiazide diuretics have also been linked to this chronic form, and there have been recent reports of lupus erythematosus tumidus induced by anti-TNF drugs such as infliximab and adalimumab and angiotensin converting enzyme inhibitors.17,87–89
DiagnosisSystemic DILE should be suspected whenever a patient on continuous therapy for months or years becomes ANA positive and develops at least one of the characteristic symptoms of idiopathic SLE. A diagnosis of DILE must be made in a patient without a history of SLE and without serious kidney or neurologic disease. Suspicion is confirmed if clinical and immunologic changes disappear once the drug is withdrawn. Most patients with systemic DILE do not fulfill the 4 diagnostic criteria of the American Association of Rheumatology for SLE. Serology is also an important diagnostic tool. Circulating AHA, particularly antibodies to the (H2A-H2B)-DNA complex are typically detected; anti-dsDNA, anti-smooth muscle, anti-RNP, anti-Ro, and anti-La antibodies are absent. Unlike idiopathic SLE, systemic DILE does not usually present with low complement levels. Standard diagnostic criteria have not been established, but a series of characteristic findings that aid diagnosis of systemic DILE have been proposed, as follows:
- 1.
Ongoing treatment (of at least 1 month) with a drug.
- 2.
No prior history of SLE before the drug was started.
- 3.
Fever, arthritis, general malaise, and muscle pain.
- 4.
Positive findings for AHAs (especially IgG antibodies to the [H2A-H2B]-DNA complex) and for anti-single-stranded DNA antibodies. Negative findings for anti-extractable nuclear antigen antibodies.
Antigen positivity tends to have resolved months after the culprit drug is discontinued. ANA-positive seroconversion in a patient being treated with a drug that can potentially trigger systemic DILE does not necessarily mean treatment must stop, as only a small percentage of cases progress to lupus. Diagnostic criteria for the subacute and chronic cutaneous forms are not well defined. Skin lesions after a challenge test with the suspected drug is a strong indicator, but this finding has seldom been reported in the literature. These diagnoses are more likely to be made if the lesions have developed for the first time and are clinically and histologically similar to those of cutaneous lupus in middle-aged or elderly patients, if they coincide with taking a medication that has been prescribed recently, and if they improve following discontinuation of the drug. The immunologic work-up in some of the DISCLE cases we have reviewed shows that anti-Ro antibodies are more prominent than AHA antibodies, whose role is more doubtful in this type of lupus.
Although the usefulness of epidermal (patch) tests in diagnosing DILE has not been evaluated, they can be helpful in differential diagnosis to rule out other early-stage toxicodermal diseases, such as Stevens-Johnson syndrome (toxic epidermal necrolysis) or DRESS syndrome (drug reaction with eosinophilia and systemic symptoms). One recent study found these tests to be useful in cases caused by carbamazepine, a drug that can also cause DILE.90
Laboratory FindingsSystemic DILEAlthough most patients are positive for at least one ANA antibody, this is not always the case.91 The ANA profile is normally homogeneous, as most of these antibodies target histones. A nonnegligible number of patients with SLE symptoms who are taking quinidine or minocycline are ANA negative. Therefore, failure to detect ANAs does not rule out a diagnosis of DILE. In the case of systemic DILE, AHAs are the most characteristic type of ANA and positivity is a key finding in this diagnosis, present in over 90% of patients. However, this finding is less common when certain drugs (e.g., minocycline or anti-TNF agents) trigger the disease.92,93 Thus, even though AHAs are highly prevalent in systemic DILE, this finding is not diagnostic: while AHA positivity is highly sensitive, the specificity is low. As mentioned, AHAs are found in 90% to 95% of systemic DILE cases and are also present in 75% of SLE and 32% of asymptomatic ANA-positive patients. AHAs are also found in some patients with rheumatoid arthritis, Felty syndrome, juvenile rheumatoid arthritis (especially when the patient is ANA positive and has uveitis), and undifferentiated connective tissue diseases.94–97 AHAs only recognize histone epitopes in chromatin or free or denatured histones. Two types have been identified. One targets simple histones (H1, H2A, H2B, H3, and H4), whereas the other reacts to the H2A-2B and H3-4 complexes.98 IgG type antibodies that react with native chromatin or the reconstituted (H2A-H2B)-DNA complex in enzyme-linked immunosorbent assays seem to provide a good marker for DILE.99 High titers of IgG-class AHAs are a widespread finding in DILE, unless the disorder is caused by procainamide, which tends to be associated with IgM-class AHAs.100 Many patients treated with drugs that cause systemic DILE are known to be AHA positive but symptom-free.101 The antibody titer normally decreases when the culprit drug is withdrawn, although several months may go by before levels become undetectable.102 Anti-dsDNA antibodies are a rare finding in systemic DILE93; therefore, positivity should lead to suspicion of idiopathic lupus. Higher anti-dsDNA titers, however, have been found in cases triggered by anti-TNF agents. Some have found that anti-dsDNA antibodies are present in 90% of DILE induced by anti-TNF. Another difference between systemic DILE and idiopathic SLE is that ANA-complement binding does not occur in the drug-induced form.103 Anti-Ro positivity may be found in cutaneous DILE but is uncommon in systemic forms.93,104 Abnormal findings on blood work-up—such as a slightly low white blood cell, normochromic normocytic anemia, low platelet counts, or a high erythrocyte sedimentation rate—are typical in systemic DILE. These abnormalities are less common in idiopathic lupus.
DISCLEThe immunologic profile of DISCLE differs from that of systemic DILE in that anti-Ro antibodies are more often present along with ANA; anti-La antibodies are also sometimes found. Some authors point out that serum AHA positivity should not be considered as a diagnostic marker in DISCLE and debate continues: Sontheimer and colleagues104 found AHA positivity in half of a 71-patient series. Anti-dsDNA findings are usually negative. Symptoms improve for most patients 8 to 12 weeks after the drug is discontinued, but anti-Ro positivity persists for around 8 months,26 although there have been reports of persistence up to 6 years after resolution of the lesions.
Chronic Cutaneous DILEMost chronic cases show ANA positivity but anti-extractable nuclear antigen and anti-dsDNA negativity; exceptions are rare.105
ANA become undetectable some 10 weeks after withdrawal of the drug. Patients with the chronic form are usually AHA-negative.106,107
TreatmentDILE is managed by discontinuing the drug that triggered the condition. Pharmacotherapy should be reserved for refractory cases, which are usually seen in patients who actually had pre-existing lupus that became exacerbated on exposure to a drug. Although cutaneous manifestations normally disappear within weeks of discontinuing the culprit drug, complete resolution can take several months, making it necessary to provide medical treatment. The cutaneous lesions, such as vasculitis, can be temporarily treated in either DISCLE or systemic DILE by prescribing systemic corticosteroids at the doses indicated for idiopathic lupus; topical corticosteroids and hydroxychloroquine can also be used. Refractory cases can be managed with immunosuppressant agents such as thalidomide, azathioprine, cyclophosphamide, or mycophenolate-mofetil. The chronic cutaneous form can also be treated with topical corticosteroids and hydroxychloroquine, a combination that is usually effective. Further treatment with systemic corticosteroids and thalidomide should be reserved for cases that remain refractory.
ConclusionDILE is similar to idiopathic lupus but occurs as a result of exposure to an increasing number of drugs. As new therapies are developed for a multitude of diseases, the incidence of this autoimmune disorder is expected to rise significantly, especially as immunomodulators gain ground in the market. Clinical and serologic manifestations normally resolve after withdrawal of the culprit medication. In addition to systemic DILE, there are 2 cutaneous forms of the disease—one chronic and the other subacute (i.e., DISCLE). Whereas the chronic form is rare, the subacute form is considered the most prevalent DILE.
Ethical DisclosuresProtection of human and animal subjects. The authors state that no experiments were performed on humans or animals for this investigation.
Confidentiality of data. The authors declare that they have followed the protocols of their hospital concerning the publication of patient data, and that all the patients mentioned were appropriately informed and gave their written informed consent.
Right to privacy and informed consent. The authors obtained informed consent from the patients and/or subjects referred to in this article. All consent forms are in the possession of the corresponding author.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Pretel M, Marquès L, España A. Lupus eritematoso inducido por fármacos. Actas Dermosifiliogr. 2014;105:18–30.