Omalizumab is a monoclonal anti-immunoglobulin E antibody currently only approved for use in severe, refractory asthma. In recent years, many authors have reported satisfactory results with omalizumab in patients with difficult-to-treat chronic urticaria. As a result, clinical trials were undertaken to broaden the indication of omalizumab to include chronic urticaria, and the drug was recently cited as a third-line treatment after selective antihistamines at high doses in a consensus document on the treatment of chronic urticaria. In this article our aim is to provide a comprehensive update on the use of omalizumab in the treatment of chronic urticaria. The structure of this biologic agent and its possible mechanisms of actions in this setting will be presented. Treatment strategies and the different dosage regimens used in the series of cases published to date will also be reviewed. Finally, we will discuss the adverse effects that may arise with treatment and the recommended strategies for minimizing the most feared effect, anaphylaxis. Based on the experience of many researchers, omalizumab is emerging as a novel treatment for certain types of spontaneous refractory chronic urticaria and has shown promising results in this setting. The drug has a good safety profile and the main limitation is its high cost.
Omalizumab es un anticuerpo monoclonal anti-IgE únicamente aprobado para su uso en el asma grave refractario. En los últimos años han sido publicados un gran número de casos clínicos de urticaria crónica de difícil manejo terapéutico que han respondido de forma adecuada al tratamiento con omalizumab. Por ese motivo, se han puesto en marcha ensayos clínicos para ampliar la indicación a esta enfermedad, y recientemente el fármaco ha sido incluido en una guía de consenso para el tratamiento de la urticaria crónica como fármaco de tercera línea después de los antihistamínicos selectivos a dosis altas. El objetivo de este artículo es realizar una actualización integral de la aplicación de omalizumab en el tratamiento de la urticaria crónica: revisaremos su estructura, discutiremos los hipotéticos mecanismos de acción en esta enfermedad y expondremos su modo de empleo y las diferentes posologías empleadas en las series de casos publicados hasta el momento. Por otro lado, también enumeraremos sus efectos secundarios y daremos las pautas de prevención a seguir para minimizar su efecto secundario más temible, la anafilaxia. En definitiva, y según la experiencia de muchos investigadores, omalizumab se perfila como un fármaco novedoso que ha mostrado resultados prometedores en algunos tipos de urticaria crónica espontánea resistente con un buen perfil de seguridad, aunque con la principal limitación de su elevado coste económico.
Chronic urticaria is characterized by the appearance of wheals lasting at least 6 weeks, sometimes accompanied by angioedema.1 While clinical diagnosis is straightforward, management of this condition is difficult owing to its multifactorial etiology and unpredictable course. Chronic urticaria affects between 0.5% and 1% of the population at some point in their lives2 and has a significant negative impact on the quality of life of affected patients, comparable to that reported by patients with severe coronary artery disease.3
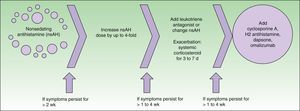
Consensus guidelines for the treatment of chronic urticaria were established in Berlin in 2008 at a joint meeting of the Dermatology Section of the European Academy of Allergology and Clinical Immunology, the Global Allergy and Asthma European Network, the European Dermatology Forum, and the World Allergy Organization4 (Fig. 1). This guide proposes the use of omalizumab, among other drugs, as a third-line treatment in cases refractory to high doses of selective antihistamines.
Treatment algorithm recommended in the EAACI/GA2LEN/EDF/WAO guideline.4
Omalizumab is a recombinant monoclonal antibody that binds to free circulating immunoglobulin (Ig) E, blocking its action at target cells. Its use has been approved by the FDA (2003) and EMA (2005) only for the treatment of moderate to severe bronchial asthma in patients aged 6 years and older. However, several recent publications have described the efficacy of omalizumab in severe chronic urticaria that is refractory to other treatments.
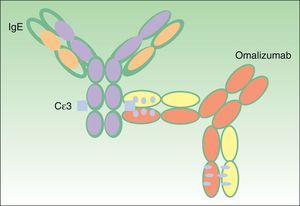
Omalizumab: Structure and FunctionStructure of OmalizumabOmalizumab is a 149-kDa humanized monoclonal antibody obtained using recombinant DNA technology. It is an IgG1 type antibody composed of human (95%) and murine (5%) fractions.5 The latter is the active fraction, and is minimized to prevent anaphylactic responses.
The antibody binds to the C¿3 domain of human IgE (Fig. 2), very close to the binding site for high and low affinity IgE receptors (Fc¿RI and Fc¿RII, respectively). Omalizumab can bind to free IgE in serum or interstitial fluids, but not to IgE molecules that are bound to the cell surface, as in this case the omalizumab binding site is occupied by the receptor.6
Mechanism of Action of OmalizumabOmalizumab has two mechanisms of action:
- -
Reduction of free IgE levels in plasma. Omalizumab binds to a region in the IgE molecule that overlaps with the site through which IgE binds to its receptor on target cells (basophils and mast cells), regardless of IgE specificity. This results in the formation of small complexes (trimers or hexamers of less than 1000 kDa) that are biologically inert (ie, do not activate complement) and are slowly eliminated via the reticuloendothelial system. In the case of high affinity receptors present on basophils and mast cells, omalizumab blocks IgE-Fc¿RI binding and prevents cellular activation and the release of vasoactive substances such as histamine and other inflammatory mediators such as leukotrienes, tryptase chymase, prostaglandin D2, and cytokines.7 These substances are responsible for the characteristic clinical features of nasal, conjunctival, bronchial, and skin hyperreactivity reactions.
- -
Indirect reduction of the number of Fc¿RI receptors in target cells.8This effect is considered secondary to the elimination of serum IgE. Previous studies have shown a strong correlation between total serum IgE levels and Fc¿RI expression on basophils in peripheral blood; receptor expression is significantly reduced when these cells are cultured in the absence of IgE.9,10 Similarly, Fc¿RI expression on basophils is greatly reduced in mature bone marrow cells exposed to a very low levels of IgE. A pharmacokinetic study demonstrated a mean decrease of 73% in Fc¿RI expression with maximum inhibition after 14 days of treatment with omalizumab.11 This reduction in the number of high affinity receptors may explain the efficacy of omalizumab in the treatment of chronic autoimmune urticaria, as the number of target receptors for anti-Fc¿RI antibodies is decreased.
However, some clinical and laboratory data suggest that the mechanism of action of omalizumab is multifactorial and that the drug may act on other, less well-known, target cells in the immune system. Sanchez-Machín and coworkers12 reported increased activity of CD4+ T lymphocytes in the serum of a patient with non-autoimmune chronic urticaria who responded rapidly to a regimen of 300 mg omalizumab every 2 weeks. Moreover, Iemoli and colleagues13 reported reduced B cell activation, decreased TNF-α and IL-4 levels, and increased IFN-γ synthesis in the serum of another patient with chronic idiopathic urticaria who was treated with omalizumab.
DosageOmalizumab is available in prefilled syringes for subcutaneous administration. It is available in 2 doses: 75 mg and 150 mg. The patient's pretreatment serum IgE level (IU/mL) and body weight (kg) are used to determine the appropriate dose (mg) and dosing frequency. The approved dose of the drug for the treatment of asthma is 0.016 mg/kg/IgE (IU/mL), provided that pretreatment IgE levels do not exceed 1500 IU/mL. The use of this omalizumab treatment regimen is associated with a reduction in IgE levels of approximately 95% from baseline in the first 3 days of treatment.14 The same dose is usually re-administered at 2 or 4-week intervals for up to 16 weeks. Treatment with the recommended dose of omalizumab produces a rapid decline in serum IgE levels to less than 50 ng/mL (20.8 IU/mL). In the treatment of allergic asthma and rhinitis, low serum IgE levels have been associated with beneficial effects, and the benefits can last up to several months or as long as IgE levels remain low.15 Omalizumab has a half-life of 26 days with an average clearance of 2.4 ± 1.1 mL/kg/d via the reticuloendothelial system. The effect of omalizumab can last for several months. There is no need to adjust the dose for age (12-76 years), race/ethnicity, or gender.
SafetyOmalizumab appears to be a safe and well-tolerated drug. Few adverse effects have been reported and most of them are minor. The most common adverse reactions reported during clinical trials were skin reactions at the injection site and urticaria. Skin reactions, such as pain, swelling, erythema, and pruritus, occur in about 40% of patients16 and urticaria is reported in 4.9% of patients.17Table 1 lists the adverse reactions, recorded in patients treated with omalizumab in clinical trials, by organ system and frequency.
Adverse Reactions Described for Omalizumab.
| Skin disorders and injection site reactions | |
| Common | Injection site reactions including swelling, pain, and pruritus |
| Uncommon | Photosensitivity, urticaria, rash, and pruritus |
| Rare | Angioedema |
| Not known | Alopecia |
| Nervous system disorders | |
| Common | Headachea |
| Uncommon | Syncope, paresthesia, daytime somnolence, and dizziness |
| Gastrointestinal disorders | |
| Very common | Feverb |
| Common | Upper abdominal pain |
| Uncommon | Dyspeptic signs and symptoms, nausea, and diarrhea |
| Immune system disorders | |
| Rare | Anaphylactic reaction and other serious allergic conditions |
| Not known | Serum sickness |
| Infections and infestations | |
| Uncommon | Pharyngitis |
| Rare | Parasitic infection |
| Blood disorders | |
| Not known | Severe idiopathic thrombocytopenia |
| Vascular disorders | |
| Uncommon | Postural hypotension, flushing |
| Disorders of the respiratory system | |
| Uncommon | Allergic bronchospasm, coughing |
| Rare | Laryngeal edema |
| Not known | Allergic granulomatous vasculitis (Churg Strauss syndrome) |
| Musculoskeletal disorders | |
| Not known | Arthralgia, myalgia, joint swelling |
| Changes in general health | |
| Uncommon | Flu-like syndrome, swollen arms, weight gain, and fatigue |
Adverse reactions are listed in order of decreasing seriousness within each frequency category. Frequency categories are defined as follows: very common (≥ 1/10), common (≥ 1/100 to < 1/10), uncommon (≥ 1/1000 to < 1/100), rare (≥ 1/10 000 to < 1/1000) and very rare (< 1/10 000). The frequency of the reactions reported in the post-marketing phase is not known (ie, cannot be estimated from available data).
Modified from the Summary of Product Characteristics for omalizumab.
The most feared adverse reaction is anaphylaxis, which is mediated by the murine fraction of omalizumab. In 2007, the American Academy of Allergy, Asthma, and Immunology created the Omalizumab Joint Task Force to record data on omalizumab-associated anaphylaxis. The Task Force found an incidence of anaphylaxis of 0.2%, with 61% of such reactions occurring within 2 hours of the first 3 injections and 14% within 30 min of the fourth or subsequent injections. In line with these observations they established the following recommendations for all patients treated with omalizumab (Table 2).18
Recommendations of the Omalizumab Joint Task Force to Avoid the Risk of Anaphylaxis.
| 1. Obtain informed consent2. Educate patients about anaphylactic reactions3. Provide patients with self-injectable adrenaline4. Observe patients for 2 h after the first 3 injections5. Observe patients for 30 min after the fourth injection |
If these recommendations are followed, 77% of anaphylactic reactions can be detected in advance and treated appropriately.19
Omalizumab was evaluated in an extensive program that included 12 Phase IIB and Phase III clinical trials involving over 5243 patients treated with omalizumab for severe IgE-mediated asthma and rhinitis. These studies concluded that the safety profile of omalizumab was comparable to that of the control group and of standard therapies with H1 antihistamines, and found no increase in the risk of cancer in omalizumab-treated patients.20 Moreover, a recent review of the incidence of primary tumors in 32 randomized, double-blind, placebo-controlled trials concluded that there was no statistically significant association between treatment with omalizumab and the development of cancer.21
The FDA is currently evaluating the provisional safety results from an ongoing study of omalizumab. This is an observational study involving approximately 5000 patients treated with omalizumab and 2500 controls; the main objective is to evaluate the long-term profile of omalizumab in patients followed up over a 5-year period.22
Omalizumab in Chronic UrticariaOmalizumab is being used successfully in the treatment of chronic urticaria, as evidenced by at least 105 published cases of patients with severe chronic urticaria who have been treated with omalizumab. Moreover, a total of 139 patients have been included in randomized studies comparing the effects of omalizumab with those of a placebo. The most relevant data from these studies are shown in Table 3. The drug has proven effective in cases of autoimmune and non-autoimmune chronic urticaria, with variable results in cases of physical and cholinergic urticaria.
Clinical Trials and Case Series of Patients With Chronic Urticaria Treated With Omalizumab.
| First Author | Year | Number of Cases | Type of Urticaria | Dose Regimen | Comments |
| Case Reports, Case Series, Non-Randomized Clinical Trials | |||||
| Boyce23 | 2006 | 1 | CU | 375 mg/2 wk | Complete response in a 12 year-old patient |
| Spector et al.24 | 2007 | 3 | 2 CIU1 ACU | Variable | Complete response in 3 patients |
| Güzelbey et al.25 | 2008 | 1 | CSU | 150 mg/4 wk | Complete response in solar urticaria |
| Godse26 | 2008 | 1 | CIU | 300 mg/4 wk | Significant improvement in patient |
| Metz et al.27 | 2008 | 1 | CCU | 150 mg/4 wk | Complete response |
| Otto et al.28 | 2009 | 1 | CCU | 300 mg/4 wk | Significant improvement |
| Magerl et al.29 | 2010 | 8 | 7 CIU1 CPU | Variable | Response in all patients; complete clinical response in 6 patients |
| Vestergaard et al.30 | 2010 | 2 | 2 CIU | Variable | Complete response |
| Krause et al.31 | 2010 | 1 | CFU | 300 mg/2 wk | Complete response |
| Waibel et al.32 | 2010 | 1 | CSU | 400 mg/2 wk | Partial response |
| Romano et al.33 | 2010 | 2 | 2 CIU | 400 mg/2 wk | Complete response |
| Bullerkotte et al.34 | 2010 | 1 | CSU | 450 mg/2 wk | Complete response |
| Sabroe35 | 2010 | 1 | CCU | 300 mg/2 wk | No response |
| Bindslev-Jensen et al.36 | 2010 | 1 | CPU | 150 mg/2 wk | Complete response associated with decreased degranulation in the basophil test |
| Al-Ahmad37 | 2010 | 3 | 3 ACU | 300 mg/4 wk | Response in all patients |
| Iemoli et al.13 | 2010 | 1 | CIU | 300 mg/2 wk | Complete response associated with decreases in TNF-α and IL-4 and increases in IFN-γ |
| Saavedra et al.8 | 2011 | 1 | CIU | 300 mg/2 wk | Satisfactory response associated with 80% decrease in Fc¿RI receptor expression |
| Groffik et al.38 | 2011 | 9 | 9 CIU | Variable | Response in all patients |
| Metz et al.39 | 2011 | 7 | 2 CSU1 HU1 CU1 CPU1 CFU | Variable | Complete response in cases of CSU, CU, CFU, and CPUNo response in case of HU |
| Godse26 | 2011 | 5 | 5 CIU | 300 mg/2-4 wk | Significant improvement in all patients |
| Sanchez-Machín et al.12 | 2011 | 1 | 1 CIU | 300 mg/2 wk | Complete response associated with increased activity of CD4+ T cells |
| Duchini et al.40 | 2011 | 1 | CSU | 150 mg/4 wk | No response |
| Buyukozturk et al.41 | 2012 | 14 | 2 AE12 CIU | Variable | Significant improvement in urticaria activity scores and in patient quality of life. |
| Ivyanskiy et al.42 | 2012 | 19 | 12 CIU6 ACU1 CPU | 150 mg/2 wk | Complete response in 11 patients, partial response in 5, and no response in 3 |
| Observational Studies | |||||
| Kaplan et al.43 | 2008 | 12 | 12 ACU | Variable | Complete response in 7 patients, significant improvement in 4, and no response in 1 |
| Ferrer et al.44 | 2011 | 9 | 9 CIU | 300 mg | Response in all patients; complete clinical response in 7 patients |
| Randomized, Placebo-Controlled Trials | |||||
| Maurer et al.45 | 2011 | 27 cases22 controls | 27 ACU | Variable | IgE antibodies against thyroperoxidaseProtection against the development of wheals in 70.4% of omalizumab-treated patients versus 4.5% of placebo-treated patients |
| Saini et al.46 | 2011 | 21 CIU25 CIU23 CIU21 CIU | 69 CIU | 600 mg300 mg75 mgplacebo | Dose-ranging study: significant improvement with 300 mg and 600 mg and no response with 75 mg. A 150-mg dose was not included in this study. |
ACU: autoimmune chronic urticaria; AE: angioedema; CCU: chronic cholinergic urticaria; CFU: chronic factitious urticaria; CIU: chronic idiopathic urticaria; CPU: chronic pressure urticaria; CSU: chronic solar urticaria; CU: cold urticaria; HU: heat urticaria.
In most published articles omalizumab has been used to treat chronic urticaria following the guidelines for the treatment of severe allergic asthma: between 75 mg and 375 mg at 2- or 4-week intervals, depending on the patient's body weight and baseline IgE levels. Only 1 clinical trial has studied the starting dose of omalizumab in the treatment of chronic non-autoimmune urticaria.46 In this study by Saini and colleagues a total of 90 patients were randomized to receive a single dose of 75, 300, or 600 mg of omalizumab or placebo. Assessment of the response at 4 weeks revealed a mean decrease in the urticaria activity score of 14.6 points in the 600-mg omalizumab group, 19.9 points in the 300-mg group, 9.8 points in the 75-mg group, and 6.9 points in the placebo group. The study demonstrated that 300 mg was the most effective dose in the treatment of urticaria, while the efficacy of the 75-mg dose was similar to that of the placebo. Moreover, a dose of 300 mg was used in most of the experimental studies in which a satisfactory clinical response was obtained. However, it should be noted that Saini and colleagues did not include a 150-mg dose of omalizumab. In a more recent study, 19 patients with chronic urticaria (63% idiopathic, 32% autoimmune) were treated with a fixed dose of 150 mg every 2 weeks; this resulted in complete resolution in 11/19 patients (58%), partial resolution in 5/19 patients (26%), and no response in only 3/19 patients (16%), suggesting that 150 mg is the minimum effective dose for the treatment of chronic urticaria.42
Saini and coworkers did not present conclusive data on maintenance doses and dosing intervals. An attempt by Romano and colleagues33 to increase the interval between doses to over 4 weeks in 2 cases of severe chronic urticaria resulted in clinical relapse and the reappearance of wheals after initial therapeutic success. However, Ferrer and colleagues44 obtained favorable clinical responses in 9 patients with doses of 300 mg omalizumab at variable intervals (monthly in 5 patients, bi-monthly in 1 patient, and even quarterly patient in 3 patients).
ConclusionsOmalizumab is a monoclonal anti-IgE antibody currently approved only for the treatment of severe asthma. Its use in the treatment of other diseases involving increases in serum IgE levels, such as atopic dermatitis47 and food allergy, has also been investigated, and several researchers have reported promising results and significant clinical improvement in severe cases of spontaneous chronic urticaria refractory to other treatments. The main mechanism of action of omalizumab in the treatment of chronic urticaria involves blocking the binding of IgE to the Fc¿RI receptor, resulting in a reduction in free plasma IgE and in the number of Fc¿RI receptors on the surface of mast cells and basophils. However, omalizumab has also been shown experimentally to act on other targets such as cellular immunity via mechanisms that remain to be elucidated. Several clinical trials have demonstrated a good safety profile for omalizumab; anaphylaxis, the most serious adverse effect, is rare and controllable if suitable preventive measures are adopted. The appropriate dosage of omalizumab in the treatment of chronic urticaria is yet to be determined; in most cases reported the doses used are the same as those recommended for the treatment of asthma, which are calculated according to the patient's weight and pretreatment serum IgE levels. However, the optimal dose may be between 150 mg and 300 mg every 2 to 4 weeks. The main limitation of this drug is its high cost, but compassionate use may be justified in cases of severe refractory chronic urticaria that cause great deterioration in the patient's quality of life.
Ethical DisclosuresProtection of persons and animalsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestDr. Laura Francés and Dr. María del Carmen Leiva declare no conflict of interest.
Dr. Juan Francisco Silvestre is a member of a Novartis Advisory Board.
Please cite this article as: Francés L, Leiva-Salinas M, Silvestre J. Omalizumab en el tratamiento de la urticaria crónica. Actas Dermosifiliogr. 2014;105:45–52.