A 33-year-old woman of Peruvian nationality was admitted to the intensive care unit with a pulmonary embolism. While admitted to the hospital, she presented multiple complications, such as consecutive episodes of sepsis due to Escherichia coli, Klebsiellapneumoniae, and Pseudomonas aeruginosa, which required hemodynamic support, mechanical ventilation, and broad antibiotic coverage. Seven weeks after admission, a dermatologic assessment was requested due to skin lesions that had appeared on the torso and later spread to the proximal part of the lower limbs. The week prior to admission, the patient had undergone surgery for a suprasellar meningioma, with no immediate complications, after which she was prescribed high-dose corticosteroid treatment.
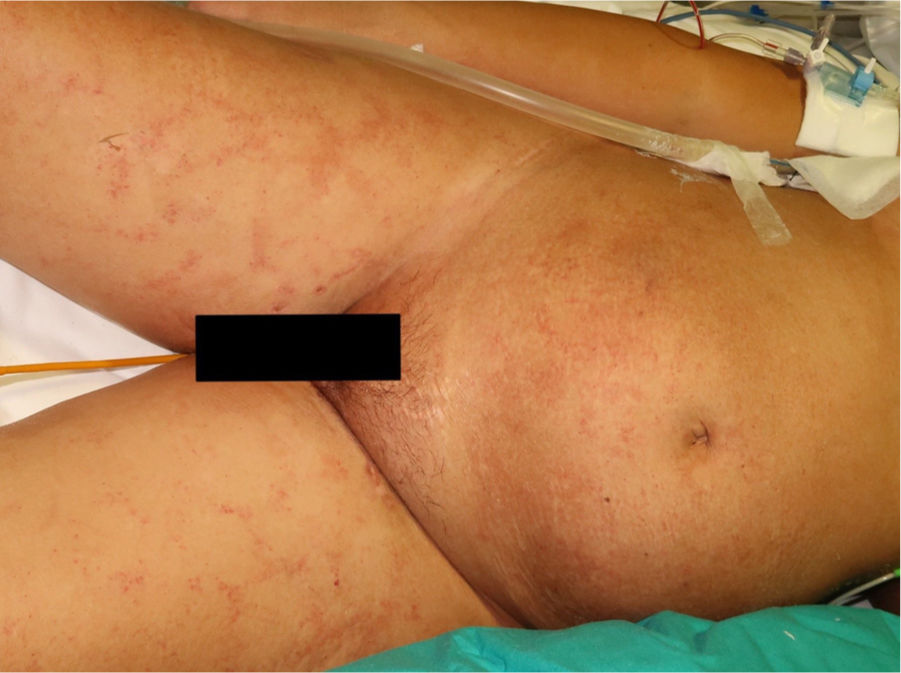
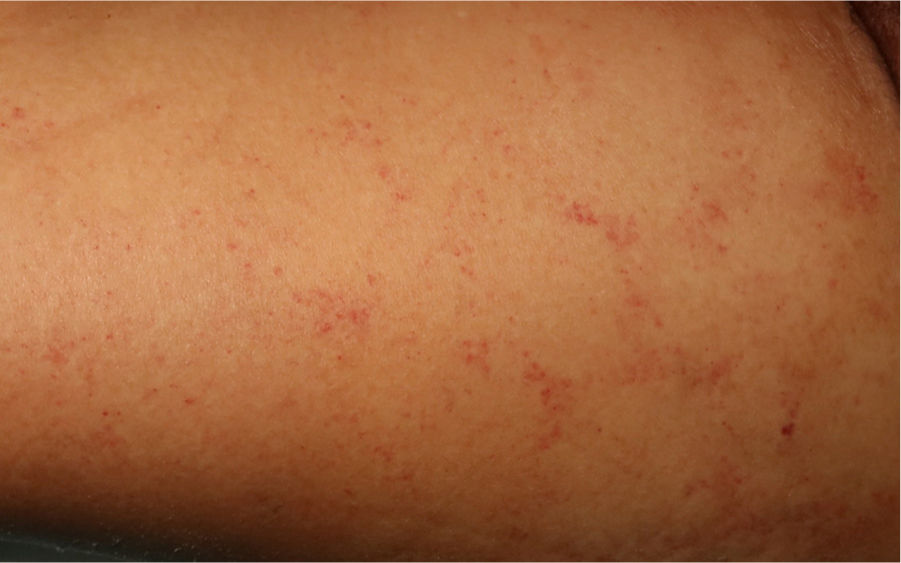
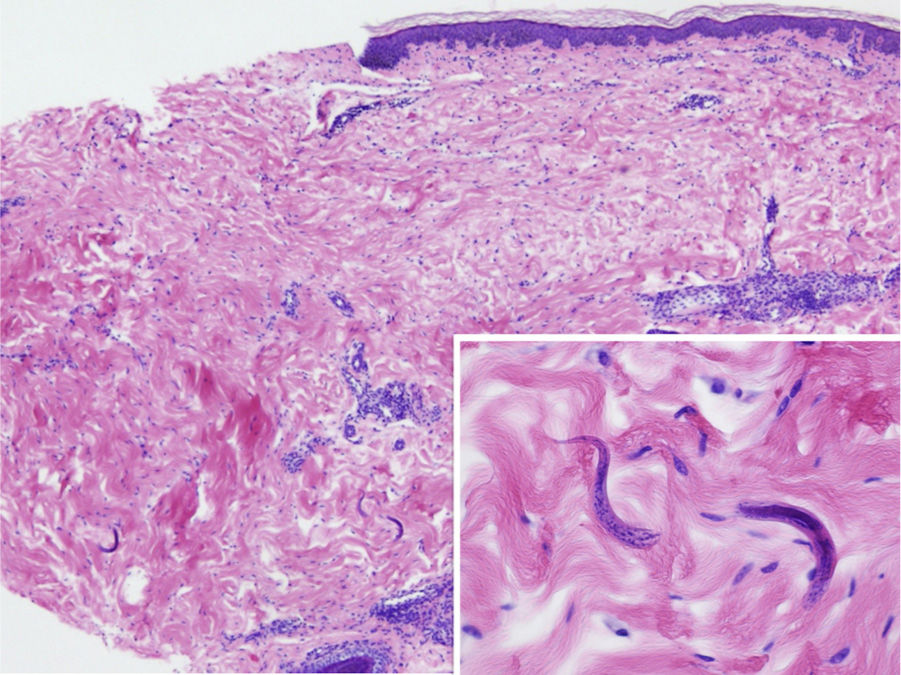
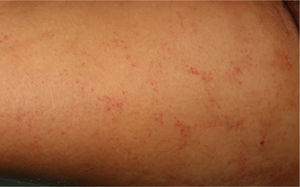
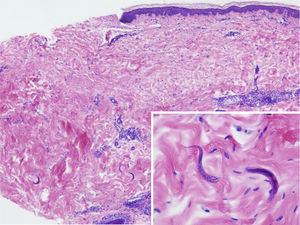
The physical examination revealed exanthema consisting of nonpalpable petechiae, grouped in a reticular pattern, in the periumbilical region and on the anterior and proximal surfaces of both thighs; the petechiae did not fade under glass (Figs. 1 and 2). Histopathology revealed a moderate perivascular infiltrate in the superficial and deep dermis, with lymphocytes and eosinophils. Moreover, elongated structures were observed in the deep dermis, among the strands of collagen, with basophilic stippling in their interior; the structures were identified as nematode larvae (Fig. 3). Blood tests revealed anemia and leukocytosis with eosinophilia, and nematode larvae were detected in the microbiologic study of the bronchoalveolar lavage.
The microorganism was identified as Strongyloides stercoralis and cultures of bronchoalveolar lavage and feces confirmed growth; the patient was therefore diagnosed with Strongyloides hyperinfestation and disseminated strongyloidiasis.
Treatment was instated with ivermectin administered via nasogastric tube. As no improvement was observed in terms of blood tests or parasite load, and in light of suspected poor digestive absorption due to the patient's critical state and paralytic ileus, the medication was then administered subcutaneously at a dosage of 200μg/kg/d, after requesting compassionate use. The parasite load fell with the treatment, but the patient never recovered from the neurologic clinical symptoms, suffered a continuous series of superinfections, and died.
Strongyloidiasis is a disease caused by the parasite S. stercoralis. This is a nematode with a global distribution, whose main reservoir is humans; it is endemic in rural areas of tropical and subtropical regions and, in Peru, it is considered to be highly endemic.1 It has 2 reproductive cycles, a free-living cycle in the soil and a parasitic cycle in the host intestine. Thus, in the filariform stage, the larvae penetrate the skin and migrate through the venous system to the lungs and the intestine, where they mature and reproduce; the eggs and larvae are eliminated by the host via the feces. They have the ability to complete their cycle in the human host, produce autoinfection, and evade the host immune response, producing chronic disease lasting decades. In this way, they rarely produce symptoms, except for nonspecific gastrointestinal symptoms accompanied by eosinophilia in blood tests. Systemic dissemination is rare and may occur in immunosuppressed patients, especially during treatment with corticosteroids, with high mortality (between 70% and 90%).2
Development of disseminated disease should be suspected in patients with a history of travel to areas where the disease is endemic (even when many years have since passed), a history of corticosteroid therapy, persistent bacteremia with organisms of enteric origin (as the parasites tend to serve as carriers for those organisms), nonspecific gastrointestinal and respiratory systems, neurological abnormalities, and concomitant infection with other intestinal parasites.3 Eosinophilia is characteristic of the disease, although it may be absent in immunosuppressed patients.
Cutaneous manifestations are rare and may appear as a purpuric petechial rash; periumbilical petechial rash is a sign of poor prognosis, previously described in the literature.4 The lesions are purpuric, nonpalpable, and take on the appearance of fingerprints, typically located in the periumbilical region and on the anterior surface of the thighs. The biopsy may show parasites around the blood vessels, with no signs of vasculitis.5
The microbiologic diagnosis is based on serology, specific culture in feces, or direct observation of the nematode. The treatment of choice is oral ivermectin at a dosage of 200μg/kg/d.6 On very specific occasions, it is necessary to use the subcutaneous presentation of this drug, especially in cases of gastrointestinal involvement secondary to hyperinfestation with obstruction or paralytic ileus, and in patients with a low level of consciousness—situations where absorption and tolerance of the orally administered drug are in question. Subcutaneous administration constitutes compassionate use and favorable results have been reported in the literature.7 Thiabendazole and albendazole are therapeutic alternatives.
As dermatologists, we must suspect strongyloidiasis in the case of a patient with periumbilical petechial rash, especially if the patient has traveled to regions where the disease is endemic and has received immunosuppressant treatment, including oral corticosteroids. It is essential to rule out strongyloidiasis by means of serological screening in at-risk patients before instating immunosuppressant or biologic treatment.8
Conflicts of InterestThe authors declare that they have no conflicts of interest.