The Bacillus Calmette-Guérin (BCG) tuberculosis vaccine contains live, attenuated strains of Mycobacterium bovis and helps prevent serious disseminated Mycobacterium tuberculosis infection.1–6 The World Health Organization recommends its use in countries endemic for tuberculosis, such as Argentina, where it is part of the national vaccination program, and, with the exception of special cases, is administered in the first month of life.1,7 Although the BCG vaccine is considered safe,2,3,5,6 there have been isolated reports of local and/or systemic manifestations.1,3,5,8,9 Immune studies are mandatory in patients with systemic manifestations, and it is particularly important to check for genetic diseases with Mendelian susceptibility to mycobacterial disease (MSMD).2,10
This article describes a case of disseminated BCG infection (BCGosis) that led to a diagnosis of MSMD with a STAT1 mutation.
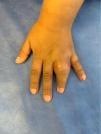
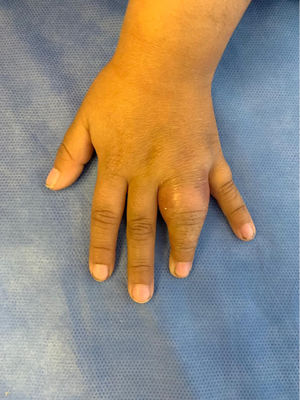
A 5-year-old girl was evaluated for a 20-day history of swelling and limited mobility of the 4th finger of the left hand accompanied by a local increase in temperature (Fig. 1). The girl, who had a twin sister, had been born at term with a low weight for her gestational age (1915g, 38 weeks). She was fully vaccinated according to the national vaccination program. At 2 years of age, she was diagnosed with lumbar scoliosis that required surgical correction at the age of 4 years. She also experienced swelling of the left elbow when 4 years old (this was interpreted and treated as cellulitis) and was hospitalized on multiple occasions for broncho-obstructive symptoms. Her twin sister and other family members had no remarkable history.
The finger lesion was diagnosed as cellulitis and treated with the oral antibiotic cephalexin. The patient, however, did not progress well and was brought in on day 4 of treatment. She was hospitalized for further investigation and intravenous antibiotic treatment. On admission, she was found to have an erythematous, crusted plaque on the right external ear, a similar lesion at the site of BCG vaccination, and a tumor consistent with a palatal torus on the hard palate.
Blood tests showed a normal complete blood count and phosphocalcic profile, elevated acute-phase reactants, and negative serology for HIV and syphilis (VDRL). Two blood cultures were negative for common pathogens. Lymphocyte populations were within normal ranges for the patient's age, with hypergammaglobulinemia of all isotypes, which was interpreted as the result of the reactive process to the infectious inflammatory condition (Table 1).
Laboratory Results.
| White blood cells | 8100/mm3 (neutrophils 73%/lymphocytes 22%/monocytes 44%) |
| Hemoglobin | 10.3g/d |
| Hematocrit | 31% |
| Platelets | 463000μL |
| C-reactive protein | 29mg/mL |
| Erythrocyte sedimentation rate | 105mm/h |
| Alkaline phosphatase | 215U/L |
| Blood cultures×2 | Negative for common pathogens |
| Lymphocyte subpopulations | CD3 65%, CD4 43%, CD8 21%, CD16/56 6%, CD19 29% |
| Serology | Negative for HIV and syphilis (VDRL) |
| IgG | 1880mg/dL |
| IgA | 367mg/dL |
| IgM | 210mg/dL |
| IgE | 502IU/mL |
Abbreviation: Ig, immunoglobulin.
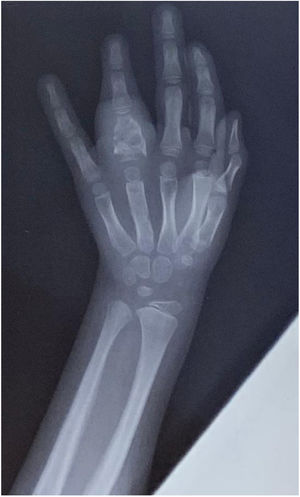
Radiography of the hands, spine, and elbows showed osteolytic lesions in the phalanx of the fourth finger of the left hand (Fig. 2) and the left elbow. Abdominal ultrasound and chest radiography showed no abnormalities. A whole-body bone scan showed increased uptake at the fourth finger of the left hand, the proximal epiphysis of the left humerus and ulna, and the right ankle.
Samples were obtained from the skin lesion on the right external ear and the fourth finger of the left hand for histologic examination and culture for common and atypical pathogens, respectively.
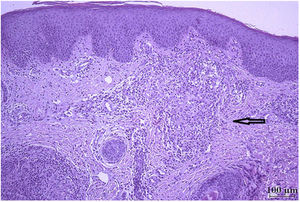
Histology showed Langhans-type multinucleated giant cells; Ziehl-Neelsen staining was negative (Fig. 3). The culture was positive for Mycobacterium, which was characterized as BCG by polymerase chain reaction analysis. The patient is currently adhering and responding well to antituberculous treatment.
Given the high clinical suspicion of an underlying immunodeficiency, it was decided to expand the immune study, with a focus on MSMD. In collaboration with the Molecular Biology and Immunology Laboratory at Hospital de Pediatría S.A.M.I.C. Juan P. Garrahan, we performed next-generation sequencing (Illumina MiSeq platform, Agilent Technologies) with a customized panel of 122 genes associated with primary immunodeficiency, including genes involved in MSMD. Sequencing detected a variant of interest in heterozygosity in STAT1 (c.469G>C, p.Glu157Gln) that was not reported in relevant databases (dbSNP [Single Nucleotide Polymorphism database], ExAC [Exome Aggregation Consortium], Ensembl [https://www.ensembl.org], Clinvar [Clinical Variant], OMIM [Online Mendelian Inheritance in Man], and HGMD [Human Gene Mutation Database]. The mutation was predicted as probably pathogenic by in silico prediction programs (SIFT, Polyphen, Mutation Taster). Other heterozygous loss-of-function variants in this gene have been implicated in MSMD.
To assess the functional impact of the variant, in collaboration with the Immunology Cell Biology Laboratory at Hospital de Pediatría S.A.M.I.C. Juan P. Garrahan, we performed a phosphorylation assay of the STAT1 protein, which showed a significant decrease in phosphorylation capacity, confirming the diagnosis of MSMD due to heterozygous STAT1 deficiency.
BCG vaccination is safe for most individuals1,3 and helps prevent disseminated tuberculosis infection.1 It can, however, cause serious, life-threatening infections (disseminated BCG infection) in immunosuppressed individuals.3 A high index of suspicion is therefore important for the early diagnosis of MSMD.
Numerous reports of disseminated BCG infection have been published,1,9,11 but few have described serious bone involvement associated with skin manifestations.3–6,9,12 The first manifestation in our patient was bone involvement, with scoliosis diagnosed at around 2 years of life that required surgical correction.
Because multiple primary and secondary immunodeficiencies can cause disseminated BCG infection,2,5,8,10,11 a full immune study is crucial in affected patients. In our case, and in collaboration with other hospital departments, suspicion of an underlying immunodeficiency led to a diagnosis of MSMD with a previously undescribed mutation in the STAT1 gene.2,8,10,11
We would like to stress the importance of multidisciplinary and interhospital collaboration, which in our case led to an accurate diagnosis of BCG infection and MSMD, enabling appropriate treatment and follow-up, improved quality of life for the patient, and appropriate genetic counseling for the patient and her family.
Conflicts of InterestThe authors declare that they have no conflicts of interest.