Two vaccines against specific human papillomavirus (HPV) types are currently being marketed. One is the bivalent vaccine Cervarix, which was authorized in 2007 for immunization against types 16 and 18, and the other is the tetravalent vaccine Gardasil, which was authorized in 2006 and also protects against types 6 and 11. The vaccines are indicated from age 9 onward for the prevention of premalignant cervical, vulvar, and vaginal lesions and cervical cancer. The tetravalent vaccine is also used to prevent the development of genital warts associated with specific HPV types. Both are administered intramuscularly and contain extracts of L1 protein from the types they protect against.1
HPV vaccination was started in the Autonomous Community of Valencia, Spain, in 2008 for girls aged 14 years. Gardasil was the vaccine funded by the National Health System and used from the outset. The supplier was changed in 2011, and the vaccine used since August of that year has been Cervarix. In January 2015, the decision was taken to bring the vaccination age forward to 12 years.
Cervarix is administered to girls aged 9-14 years in 2 doses, and the second dose must be administered 5-13 months after the first one; if the dose is administered before 5 months, then a third dose is recommended. Gardasil can be administered to girls aged 9-13 years in 2 doses (0 and 6 months) and to girls aged ≥14 years in 3 doses (0.5mL at 0, 2, and 6 months). Neither of the vaccines is recommended for girls younger than 9 years owing to the paucity of data on safety and immunogenicity in this age group.1
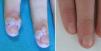
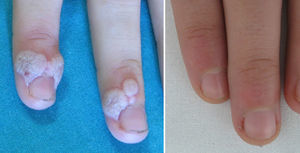
Involution of common warts has been described anecdotally after the administration of tetravalent vaccine.2,5–8Table 1 summarizes the clinical data of 5 girls whose common warts remitted after administration of the HPV vaccine. All 5 girls were prepubertal (mean age, 11.6 years) and presented multiple large warts that had appeared at least 6 months previously and were refractory to the usual topical medications. An interdepartmental consultation was made with primary care pediatricians, who were recommended to vaccinate the girls against HPV, thereby bringing the official vaccination calendar of the Autonomous Community of Valencia forward by 1-5 years. The warts resolved a mean of 11 weeks after the administration of the first dose (range, 2-28 weeks) (Fig. 1).
Patient Clinical Data.
| Sex | Age, y | Site | Time Since Diagnosis | Previous Therapy | Vaccine | Doses | Outcome After Vaccination |
|---|---|---|---|---|---|---|---|
| Female | 14 | Hands, elbows, knees | 6 mo | Cryotherapy (×6) and salicylic acid | Gardasil | 3 | Disappearance 1 mo after the third dose (28 wk) |
| Female | 12 | Hands, knees | 9 mo | Cryotherapy (×5) and salicylic acid | Cervarix | 3 | Disappearance 1 wk after the second dose (5 wk) |
| Female | 9 | Hands | 8 mo | Cryotherapy (×6) and salicylic acid | Cervarix | 2 | Disappearance 1 mo after the second dose (8 wk) |
| Female | 10 | Hands, knees | 8 mo | Cryotherapy (×8) and salicylic acid | Cervarix | 2 | Disappearance 2 wk after the first dose (2 wk) |
| Female | 13 | Hands, chest, knees | 3 y | Cryotherapy (×7) and salicylic acid | Cervarix | 2 | Disappearance 3 mo after the first dose (12 wk) |
Given the excellent response in all 5 cases, we recommended bringing the HPV vaccination age forward in specific cases of girls with recalcitrant common warts. Remission of the warts was achieved in all cases, irrespective of the vaccine used.
Clinical observations and laboratory studies have shown that currently marketed HPV vaccines induce potent activation of the immune response in almost all cases, even in immunosuppressed patients, with antibody titers up to 11-fold higher than those achieved spontaneously.2–7
Similarly, several clinical trials have shown that each vaccine provides a different degree of cross-protection against other types of HPV that are not covered by both vaccines, thus enabling a protective efficacy against HPV that was greater than expected.2–5
The immune response to HVP is strongest after 7 months and optimal in girls aged 9-11 years, although the response rate declines with age.8 Thus, the immunoglobulin G titers generated after 2 doses of vaccine in girls aged 9-14 years are not inferior to those generated after 3 doses in women aged 15-25 years; hence, only 2 doses are usually administered in girls aged less than 14 years.1 In this sense, a possible effect of sex hormones on the cellular response induced by these vaccines has been suggested, since they affect expression of HPV proteins.8
Finally, bringing the vaccination age forward to 9 years would not reduce medium- to long-term efficacy, since the bivalent vaccine has shown seropositivity rates >98% at 8 years after administration of the first dose.1
Complete remission of common warts after administration of the recombinant bivalent HPV vaccine had not been previously reported in the literature. Bringing the HPV vaccination age forward could prove to be a very useful option for the management of recalcitrant common warts in prepubertal girls. In the coming years, the incidence of common warts could decrease in women vaccinated against HPV.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Martín JM, Escandell I, Ayala D, Jordá E. Remisión espontánea de verrugas recalcitrantes en niñas tras vacunación frente al virus del papiloma humano. Actas Dermosifiliogr. 2016;107:533–535.