Leukemia cutis, which is the infiltration of leukocytes into the skin, is rare and accounts for just 3.1% of all types of leukemia. The subtypes that most commonly affect the skin are monocytic acute myeloid leukemia (AML-M5 [French-American-British classification]) and acute myelomonocytic leukemia (AML-M4), with a respective prevalence of 33% and 13% to 18%). Myeloid sarcoma, formerly known as granulocytic sarcoma, is an extramedullary tumor composed of immature myeloid cells.1 Although the skin is among the organs most frequently affected by myeloid sarcoma, this tumor is still a rare variant of leukemia cutis. We report the case of a patient with myeloid sarcoma in the area of a skin flap.
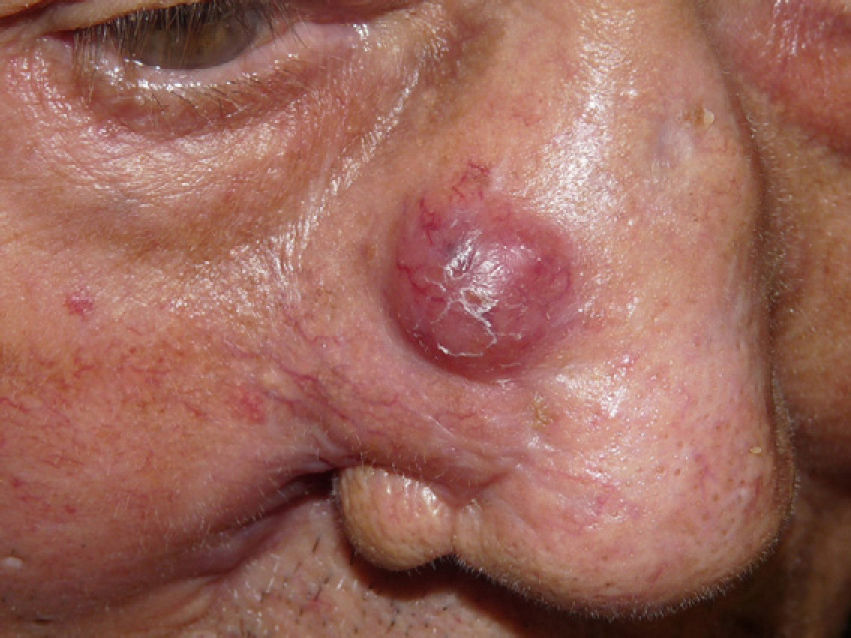
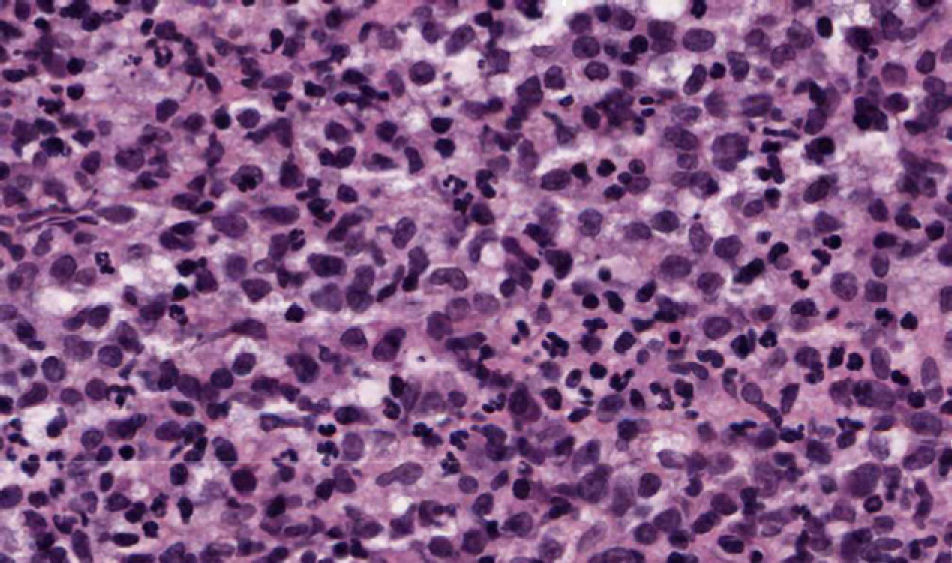
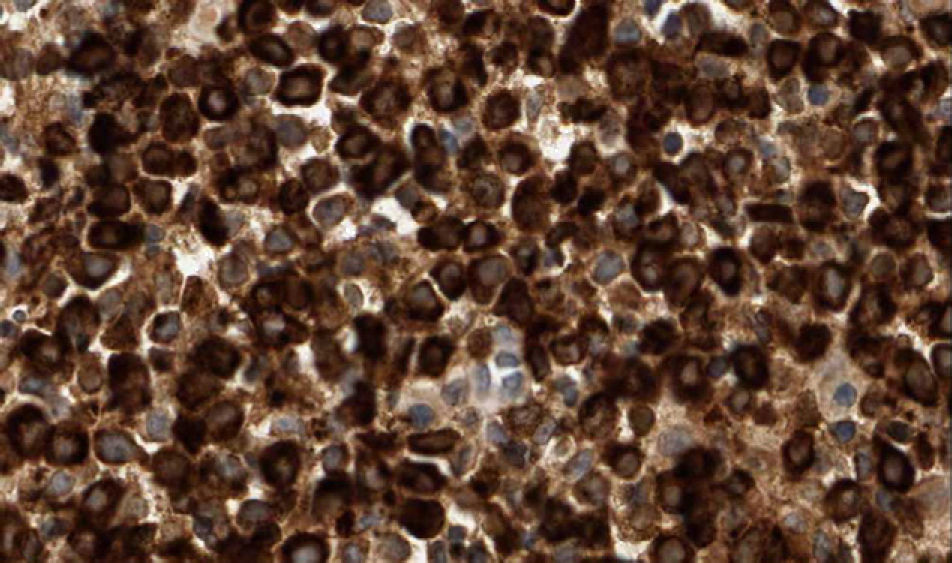
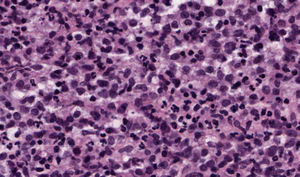
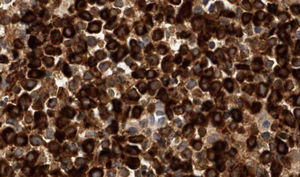
The patient, an 86-year-old man, had been diagnosed with myelodysplastic syndrome 2 years earlier. He had consulted for 2 basal cell carcinomas, one on the left temple and the other on the right ala nasi. Both tumors were excised. The surgical defect was repaired by direct closure in the first case and with a nasolabial fold flap in the second. A few hours after surgery, the patient presented at the emergency department with diffuse hemorrhage from both surgical wounds and a large hematoma in the area of the skin flap. Laboratory tests showed a leukocyte count of 12 200/μL (upper limit of normal, 10 000/μL; neutrophils, 31.2%; lymphocytes, 21.3%; monocytes, 43.6%); a hemoglobin level of 11.7g/dL (lower limit of normal, 13g/dL), a platelet count of 65 000/μL (lower limit of normal, 150 000/μL), and a creatinine level of 1.5mg/dL (upper limit of normal, 1.3mg/dL). The other tests (coagulation studies, liver function tests, and lactate dehydrogenase) were normal. One month after surgery, a fast-growing asymptomatic nodule appeared in the area of the skin flap, accompanied by progressive infiltration of the lower part of the flap (which had healed perfectly) and ulceration (Fig. 1). Biopsy of the nodule and the infiltrated scar revealed a diffuse neoplastic proliferation of medium-sized, round/oval mononuclear cells with eosinophilic cytoplasm and basophilic nuclei in the dermis and adipose tissue (Fig. 2). Immunohistochemical staining showed diffuse positivity for myeloperoxidase (MPO), CD68, and CD43, and focal positivity for CD34, leading to a diagnosis of myeloid sarcoma (Fig. 3). During this time, the patient's myelodysplastic syndrome progressed to AML-M4. Palliative treatment was initiated with thioguanine, but the patient died 3 weeks later.
Myeloid sarcoma presents as one or more tumor masses composed of immature myeloid cells. While the tumors can affect any part of the body except the bone marrow, the most common sites of involvement are bone, periosteum, skin, gums, and lymph nodes. Multifocal disease is seen in less than 10% of cases. Myeloid sarcoma can precede, coincide with, or indicate recurrence of AML. It can also indicate blastic transformation of a myelodysplastic syndrome, chronic myeloid leukemia, or other myeloproliferative disorders. It is slightly more common in men than in women (male to female ratio, 1.42:1) and in advanced ages (mean age at diagnosis, 56 years). Myeloid sarcoma of the skin presents as a solitary tumor that grows in a matter of days or weeks and typically affects the face, the scalp, or the trunk. There have also been reports of multiple and even disseminated lesions.1–4 The literature contains approximately 20 reports of myeloid sarcoma in leukemia cutis, arising at sites of previous skin lesions or trauma, mainly at central venous catheterization sites (11 cases)4 and puncture sites for venous and arterial sampling and bone marrow aspiration (4 cases).5 There have also been isolated reports of myeloid sarcoma at sites of extravasation of chemotherapy agents,6 traumatic scars and excoriations,8 Mantoux test injection sites,3 decubitus ulcer, Sister Mary Joseph's nodule, pyoderma gangrenosum,9 tetanus booster injection sites,10 and within a basal cell carcinoma.11 We reexamined the surgical specimens from the basal cell carcinomas removed from the patient's temple and nose but found no evidence of leukemic infiltration. Myeloid sarcoma in a patient with AML is normally a marker of recurrence and rapid disease progression. Of 4 such cases described in the literature, it was aleukemic in just 1 case7; in the other 3, its appearance coincided with transformation of a myelodysplastic syndrome into AML. Prognosis is generally very poor. It is noteworthy that while text books state that leukemia cutis can occur at the site of surgical scars, we found no such cases reported in the literature.
Histologic diagnosis of myeloid sarcoma requires a high level of clinical suspicion and diagnosis might be missed if there is no previous history of AML. Histologic findings include dense neoplastic infiltration of the dermis and adipose tissue, typically most intense around vessels and adnexa, without epidermotropism. The papillary dermis is usually spared (Grenz zone). Cytology varies greatly according to the origin of the tumor and the degree of cell maturation. Immunohistochemical studies are essential for diagnosis, with CD68, MPO, CD43, CD3, CD20, and chloroacetate esterase staining recommended.2,3
The pathogenesis of leukemia cutis and myeloid sarcoma is not clear, but it appears to be influenced by both the type of leukemia and local factors. Infiltration would appear to be more common in monocytic variants because neoplastic monocytes have a greater capacity to adhere to vessel walls and invade extravascular spaces, forming skin tumors.4,8 Nevertheless, local skin trauma of any type can activate keratinocytes or fibroblasts in old lesions, releasing chemotactic factors for leukocytes and inflammatory cells that would lead to the recruitment of leukemic cells.8,9 Of particular interest in the case presented is the fact that the leukemic infiltration was confined to the area of the skin flap, ie, it did not affect the surgical defect repaired by direct closure. This might be because skin flap surgery causes greater tissue damage than direct closure, and this damage, combined with the profuse hemorrhage and subsequent hematoma (related to the patient's underlying disease), would have caused greater local inflammation, which, in turn, would have promoted the recruitment of leukemic cells in a process that coincided with the transformation of myelodysplastic syndrome to AML.
Please cite this article as: García-Arpa M, et al. Sarcoma mieloide en un área de plastia. Actas Dermosifiliogr.2011;102:737-739.