Keratoacanthoma is currently considered to be an in situ squamous cell carcinoma that mainly affects patients over 70 years of age. The tumor has a good prognosis and, in some cases, can resolve spontaneously. Treatment involves simple excision. However, since the tumors generally occur on the face or extremities and display rapid growth, aggressive surgery may be required and the cosmetic results may be poor.
ObjectiveThe primary study objective was assessment of the efficacy of presurgical intralesional methotrexate infiltration to reduce the size of the tumor and the corresponding surgical defect.
Material and methodsA prospective, randomized study was undertaken in patients with a diagnosis of keratoacanthoma of at least 1.5cm who were seen in our service between January 2009 and January 2010. Two groups were established: one receiving a single infiltration of methotrexate prior to surgery and another that did not receive methotrexate.
ResultsOf the 25 patients included in the study, 10 received neoadjuvant intralesional methotrexate (group A) and 15 underwent surgery without prior infiltration of methotrexate (group B). The patients in group A displayed a reduction of between 50% and 80% in the size of the lesion prior to surgery. No complications were observed either in relation to methotrexate infusion or surgery. In group B, only 1 patient had a slight reduction in the dimensions of the lesion prior to surgery. In the remaining cases, the lesions remained similar (4 cases, 26%) or had increased in size (10 cases, 66%) at the time of surgery. Five patients in this group required hospital admission following surgery.
ConclusionsNeoadjuvant intralesional methotrexate is well tolerated and reduces the need for aggressive surgery in elderly patients with keratoacanthoma measuring more than 1.5cm on the face or extremities.
El queratoacantoma es considerado hoy día un carcinoma epidermoide in situ que aparece principalmente en pacientes mayores de 70 años. Se trata de un tumor de buen pronóstico que, en algunos casos, muestra resolución espontánea.
El tratamiento de este tipo de tumoración es la exéresis simple. Sin embargo, la localización preferente en las regiones facial y acral, el tamaño y su rápido crecimiento son factores que hacen que la cirugía sea en algunos casos agresiva y antiestética.
ObjetivoEl objetivo principal del estudio es evaluar la eficacia de la infiltración intralesional de metotrexato en la reducción del tamaño prequirúrgico de la lesión y del correspondiente defecto quirúrgico resultante de la intervención.
Material y métodosSe realizó un estudio prospectivo aleatorizado en el que se incluyeron todos aquellos pacientes atendidos en nuestro Servicio diagnosticados de queratoacantoma de al menos 1,5cm de tamaño entre enero de 2009 y enero de 2010. Se establecieron dos grupos, uno en el que los pacientes recibieron una infiltración de metotrexato previamente al acto quirúrgico y otro en el que se realizó directamente la cirugía.
ResultadosDe los 25 pacientes incluidos en el estudio, 10 casos recibieron neoadyuvancia con metotrexato intralesional (grupo A) y 15 casos fueron intervenidos mediante cirugía aislada (grupo B).
Los pacientes del grupo A mostraron una reducción en el tamaño tumoral en el momento de la cirugía que osciló entre un 50 y un 80%. Ninguno de los pacientes presentó complicaciones relacionadas con la inoculación del metotrexato ni con la intervención quirúrgica.
En el grupo B sólo uno de los casos mostró una discreta disminución de sus dimensiones en el momento del acto quirúrgico. El resto de las lesiones mostraron una estabilidad (4 casos; 26%) e incluso un aumento de las dimensiones del tumor (10 casos; 66%) en el momento de la intervención. Cinco de los casos incluidos en este último grupo requirieron ingreso hospitalario en relación con la intervención quirúrgica.
ConclusionesEl metotrexato intralesional como terapia neoadyuvante es una medida bien tolerada, que permite evitar cirugías agresivas en pacientes de edades avanzadas que presentan un queratoacantoma de diámetro superior a 1,5cm localizado en la región facial y acral.
Keratoacanthoma is a skin tumor that typically presents as a solitary lesion on the sun-exposed skin of elderly patients. It is clinically characterized by rapid growth, followed on occasions by a period of partial involution, and more rarely by a period of complete involution.1,2 It has characteristic histologic features consisting of a keratin-filled crater surrounded by a proliferative atypical squamous epithelium. While there is sufficient evidence to suggest that keratoacanthoma is a clinically distinct variant of well-differentiated squamous cell carcinoma, this is still a subject of debate.1,2
Even though involution may occur in certain cases, the fact that keratoacanthoma has been classified as a well-differentiated squamous cell carcinoma with metastatic potential means that treatment must be definitive.
The current treatment of choice is surgical excision, using either conventional surgery1,2 or Mohs micrographic surgery with fresh tissue.3,4 Alternative treatments are systemic retinoids,5 radiotherapy,6,7 curettage and electrodesiccation,8–10 intralesional 5-fluorouracil,11–14 and intralesional methotrexate.15–24 Intralesional interferon alpha-2b25 and topical imiquinoid26 have also been used, but less frequently.
Keratoacanthoma is often located in areas in which surgical excision carries considerable risk in terms of functional or cosmetic impairment due to the size of the tumor.
The use of neoadjuvant therapy to reduce tumor size prior to surgery would thus simplify the procedure and offer better functional and cosmetic outcomes.
The main aim of the current study was to assess the efficacy of intralesional methotrexate to reduce the size of tumors prior to surgery and, therefore, also the size of the corresponding surgical defects. Secondary objectives were to assess the need for complex reconstruction procedures, tolerance of methotrexate, and complications due to the administration of intralesional methotrexate.
Material and MethodsWe performed a prospective, single-center, double-blind, parallel study without placebo control. Included were patients diagnosed, both clinically and histologically, with keratoacanthoma at the Department of Dermatology of the Fundación Instituto Valenciano de Oncología in Valencia, Spain between January 1, 2009 and February 1, 2010.
Inclusion CriteriaThe inclusion criteria were as follows: a) age over 18 years, b) tumor location in the facial or acral regions, c) tumor size over 1.5cm, and d) absence of liver, blood, or kidney disorders.
The study was approved by the hospital ethics committee and written informed consent was obtained from all participants.
Study GroupsPatients were randomly assigned to 2 groups (A and B) at the baseline visit using sheets of paper stating A or B contained in sealed envelopes prepared by an observer not involved in the study. Patients in group A were treated with intralesional methotrexate and surgery and those in group B were treated with surgery only. All of the patients underwent surgery within 30 to 35 days of the baseline visit.
A case report form was completed for each patient to record key clinical and surgical information and any adverse events attributed to the administration of intralesional methotrexate. Photographs were also taken at the baseline visit and at the time of surgery to analyze changes in tumor size and appearance.
Case Report FormThe case report form contained 4 sections. The first section was used to record the patient's personal details (age and sex), and the second to record the clinical features of the tumor at the baseline visit (time since onset, location, and size). A photograph was also taken of the tumor at this time.
The third section was used to record details of the administration of intralesional methotrexate. These included the volume of methotrexate required to completely change the color of the tumor to yellow and a record of whether or not the patient had experienced discomfort during the administration of the drug.
The fourth section was used to record surgical data, namely the size of the tumor (cm), the size of the surgical defect (cm), and the technique used to repair the defect (direct closure, flap, or graft). Photographs taken on the day of the operation to record the size of the lesion and the surgical defect were also included in this section.
Study VariablesThe main study outcome measure was the reduction in tumor size achieved with intralesional methotrexate. This was assessed on the basis of the information contained in the case report forms. Secondary measures were between-group differences in surgical defect size and the proportion of patients who required complex reconstruction procedures or hospitalization, or who experienced complications.
Intralesional Administration of MethotrexateLaboratory TestsAll the patients in group A underwent a laboratory workup (complete blood count [CBC] and liver and kidney function tests) prior to the administration of methotrexate. The tests were repeated 7 days after administration to check for possible systemic complications.
Material Used in Neoadjuvant TherapyWe administered a solution of injectable methotrexate supplied in 40-mL vials containing 1000mg of the drug (25mg/mL of methotrexate in an aqueous excipient solution of sodium chloride, sodium hydroxide, and hydrochloric acid). These vials are stable at room temperature and, if handled in aseptic conditions and protected from light, can be used for 3 years after opening.
The methotrexate was injected into the tumors using 5-mL syringes and 30-gauge needles.
Injection MethodThe methotrexate was injected into the base of the tumor until this acquired a yellowish color (Fig. 1).
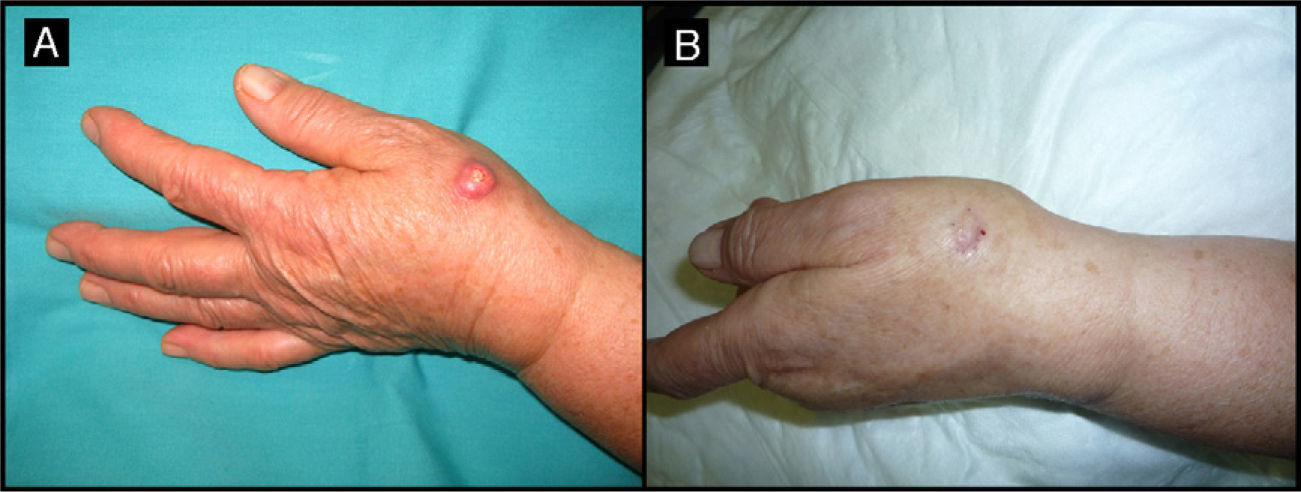
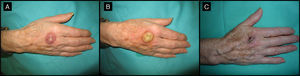
Eighty-year-old woman with a 4-month-old tumor that was histologically and clinically consistent with keratoacanthoma (patient 5, Table 1). A, 2.5-cm tumor on the back of the right hand. B, Tumor after treatment with intralesional methotrexate; note the yellow color. C, Tumor, now measuring 1cm, at the time of surgery.
Adverse events due to the administration of intralesional methotrexate were recorded in the case report forms. We evaluated complications associated with the injection technique and with the drug. In the first case, we recorded whether or not the patient experienced discomfort during the injection and also noted any signs of necrosis or other local complications. The presence of possible systemic complications due to methotrexate was evaluated by laboratory tests (CBC, liver and kidney function tests, lipid profile, and urine sediment test).
Assessment of Results and Statistical AnalysisThe case report forms, which included photographic records, were evaluated by an external investigator who was not involved in the conduct of the study or the randomization of the patients to the treatment groups.
Because keratoacanthoma is a rare disease, rather than calculate a minimum sample size, we decided to recruit all patients diagnosed with keratoacanthoma within a specific period (January 2009 to February 2010).
Quantitative variables were compared using the t test and qualitative variables using contingency tables and the χ2 or Fisher test. Statistical significance was set at P<.05. The statistical analysis was carried out using SPSS version 15.0
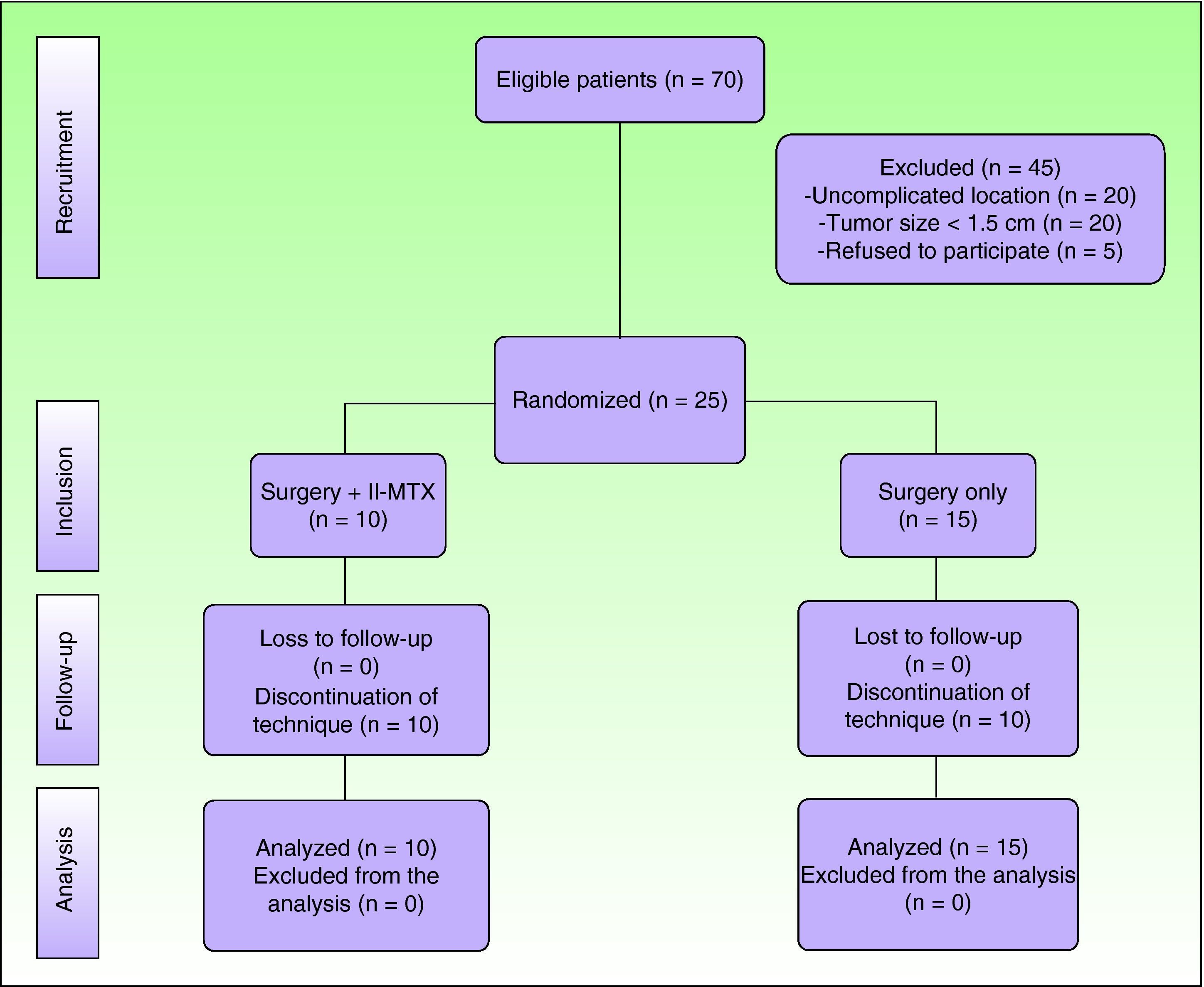
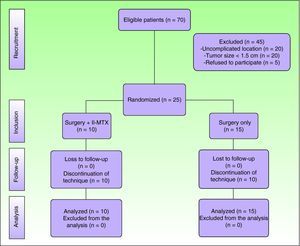
ResultsSeventy patients were clinically and histologically diagnosed with keratoacanthoma during the study period. Of these, 25 (36%) met the inclusion criteria. The remaining 45 patients were excluded because they had a tumor size of less than 1.5cm (n=20), because the tumor was not located in the facial or acral regions (n=20), or because they refused to participate in the study (n=5). (Fig. 2)
The 25 patients included were randomly allocated to group A (neoadjuvant intralesional methotrexate treatment group, n=10) or group B (surgery-only group, n=15).
The characteristics of the patients in each group are shown in Tables 1 (group A) and 2 (group B).
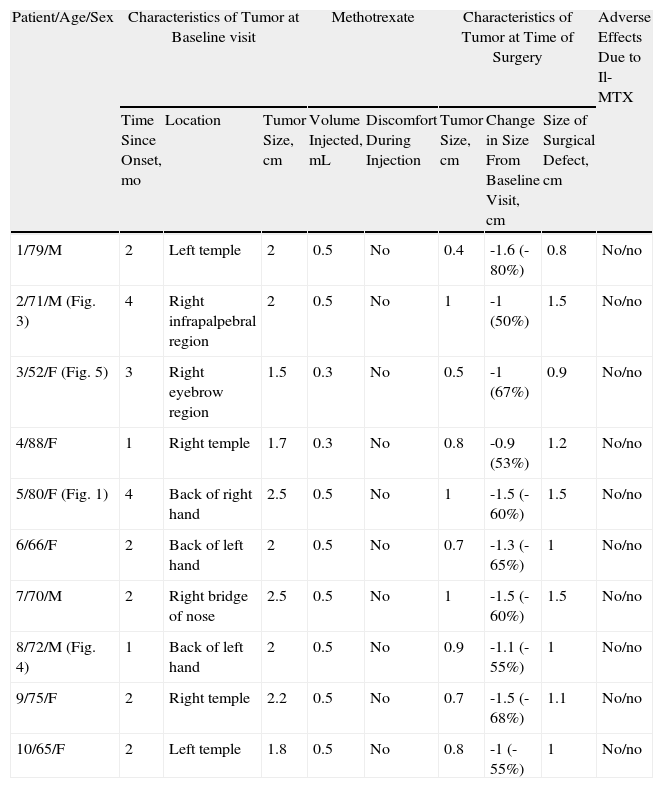
Characteristics of Patients Treated With Intralesional Methotrexate (Il-MTX) Before Surgery (Group A).
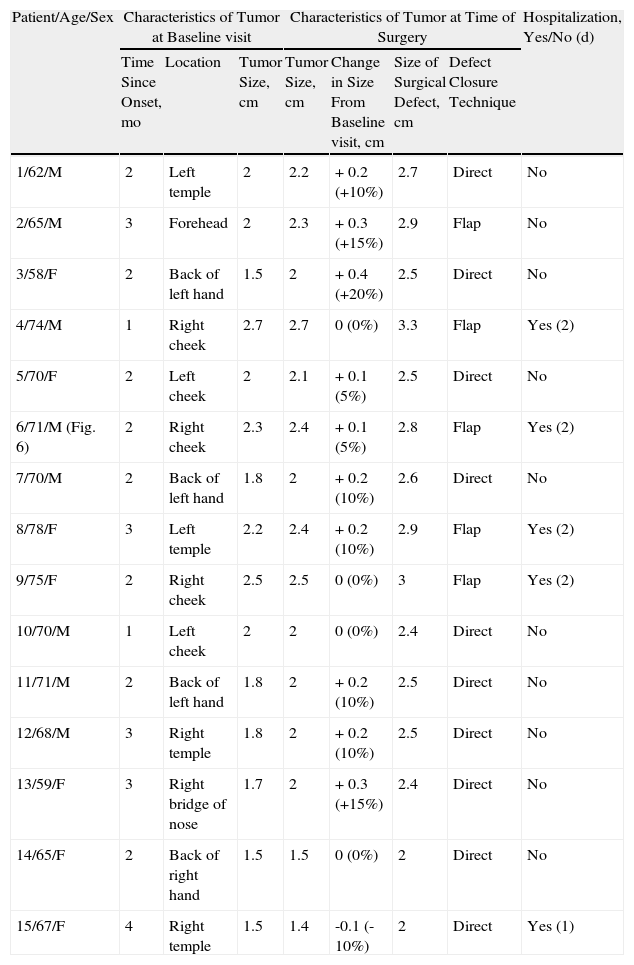
| Patient/Age/Sex | Characteristics of Tumor at Baseline visit | Methotrexate | Characteristics of Tumor at Time of Surgery | Adverse Effects Due to Il-MTX | |||||
| Time Since Onset, mo | Location | Tumor Size, cm | Volume Injected, mL | Discomfort During Injection | Tumor Size, cm | Change in Size From Baseline Visit, cm | Size of Surgical Defect, cm | ||
| 1/79/M | 2 | Left temple | 2 | 0.5 | No | 0.4 | -1.6 (-80%) | 0.8 | No/no |
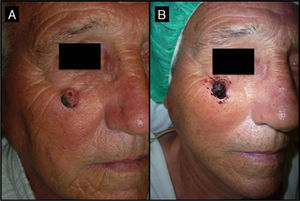
| 2/71/M (Fig. 3) | 4 | Right infrapalpebral region | 2 | 0.5 | No | 1 | -1 (50%) | 1.5 | No/no |
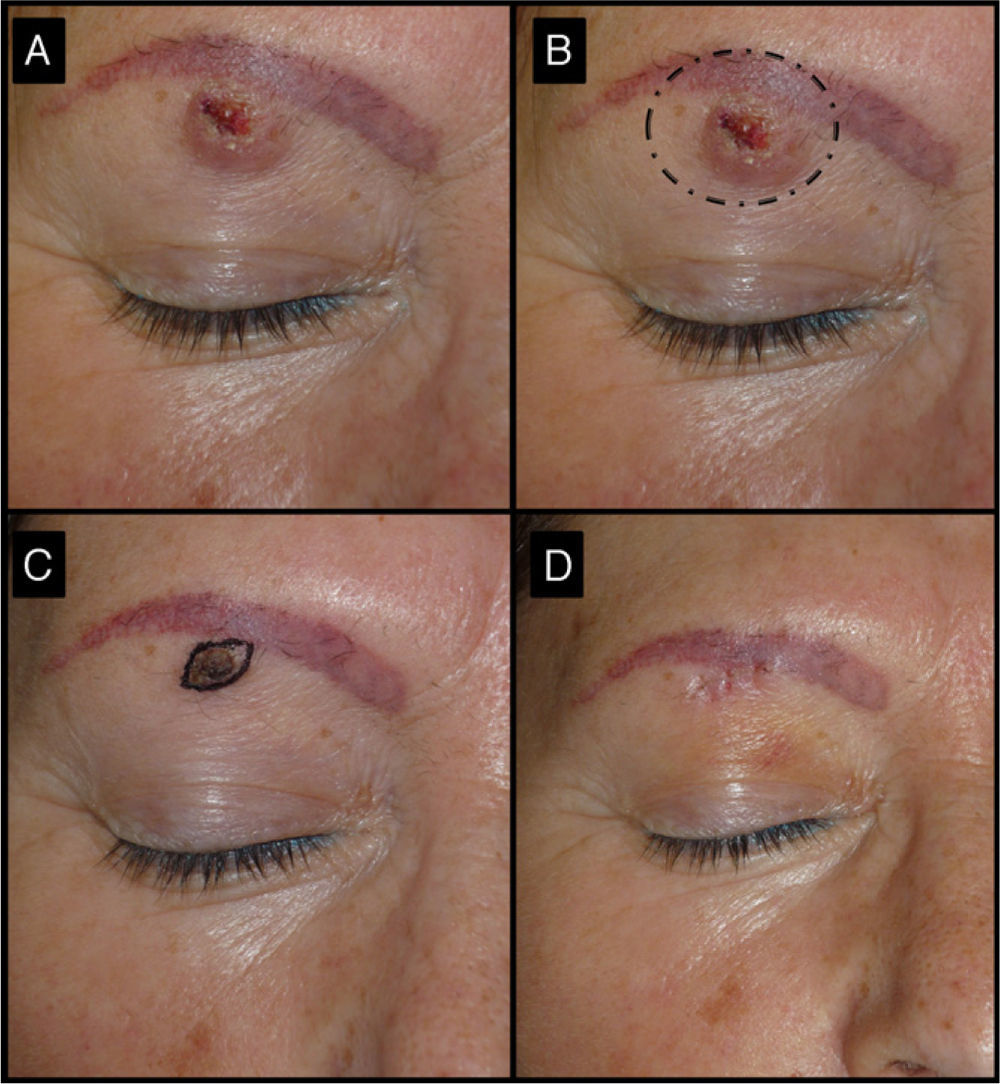
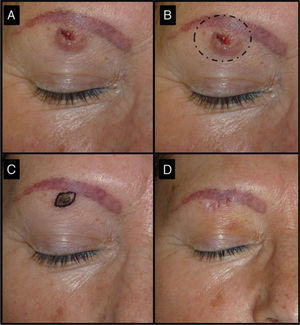
| 3/52/F (Fig. 5) | 3 | Right eyebrow region | 1.5 | 0.3 | No | 0.5 | -1 (67%) | 0.9 | No/no |
| 4/88/F | 1 | Right temple | 1.7 | 0.3 | No | 0.8 | -0.9 (53%) | 1.2 | No/no |
| 5/80/F (Fig. 1) | 4 | Back of right hand | 2.5 | 0.5 | No | 1 | -1.5 (-60%) | 1.5 | No/no |
| 6/66/F | 2 | Back of left hand | 2 | 0.5 | No | 0.7 | -1.3 (-65%) | 1 | No/no |
| 7/70/M | 2 | Right bridge of nose | 2.5 | 0.5 | No | 1 | -1.5 (-60%) | 1.5 | No/no |
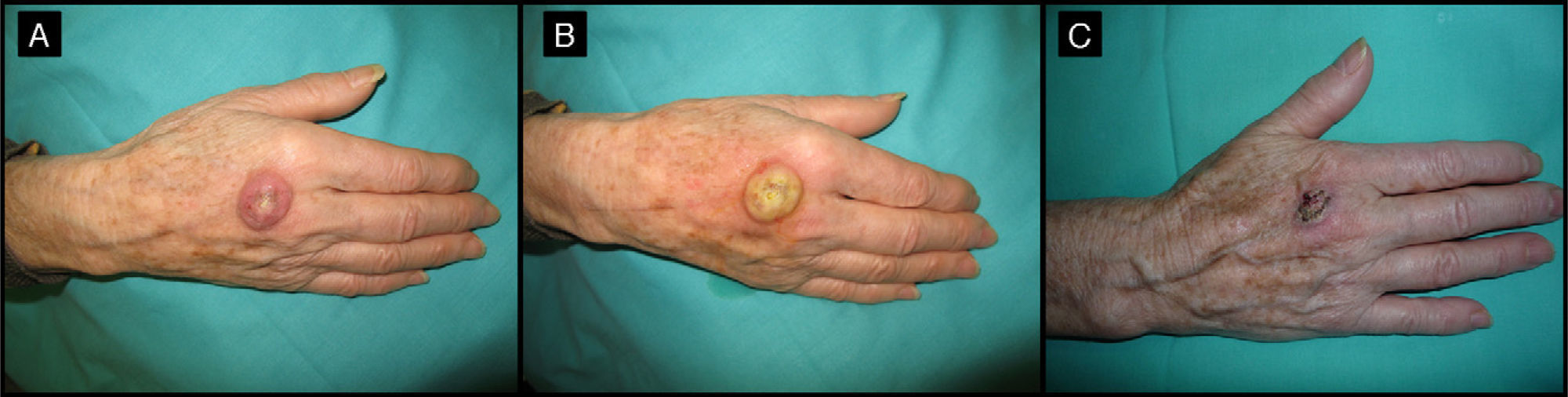
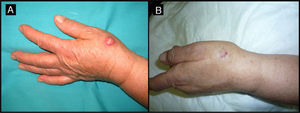
| 8/72/M (Fig. 4) | 1 | Back of left hand | 2 | 0.5 | No | 0.9 | -1.1 (-55%) | 1 | No/no |
| 9/75/F | 2 | Right temple | 2.2 | 0.5 | No | 0.7 | -1.5 (-68%) | 1.1 | No/no |
| 10/65/F | 2 | Left temple | 1.8 | 0.5 | No | 0.8 | -1 (-55%) | 1 | No/no |
Abbreviations: F, female; M, male.
Characteritsics of Patients Treated With Surgery Only (Group B).
| Patient/Age/Sex | Characteristics of Tumor at Baseline visit | Characteristics of Tumor at Time of Surgery | Hospitalization, Yes/No (d) | |||||
| Time Since Onset, mo | Location | Tumor Size, cm | Tumor Size, cm | Change in Size From Baseline visit, cm | Size of Surgical Defect, cm | Defect Closure Technique | ||
| 1/62/M | 2 | Left temple | 2 | 2.2 | + 0.2 (+10%) | 2.7 | Direct | No |
| 2/65/M | 3 | Forehead | 2 | 2.3 | + 0.3 (+15%) | 2.9 | Flap | No |
| 3/58/F | 2 | Back of left hand | 1.5 | 2 | + 0.4 (+20%) | 2.5 | Direct | No |
| 4/74/M | 1 | Right cheek | 2.7 | 2.7 | 0 (0%) | 3.3 | Flap | Yes (2) |
| 5/70/F | 2 | Left cheek | 2 | 2.1 | + 0.1 (5%) | 2.5 | Direct | No |
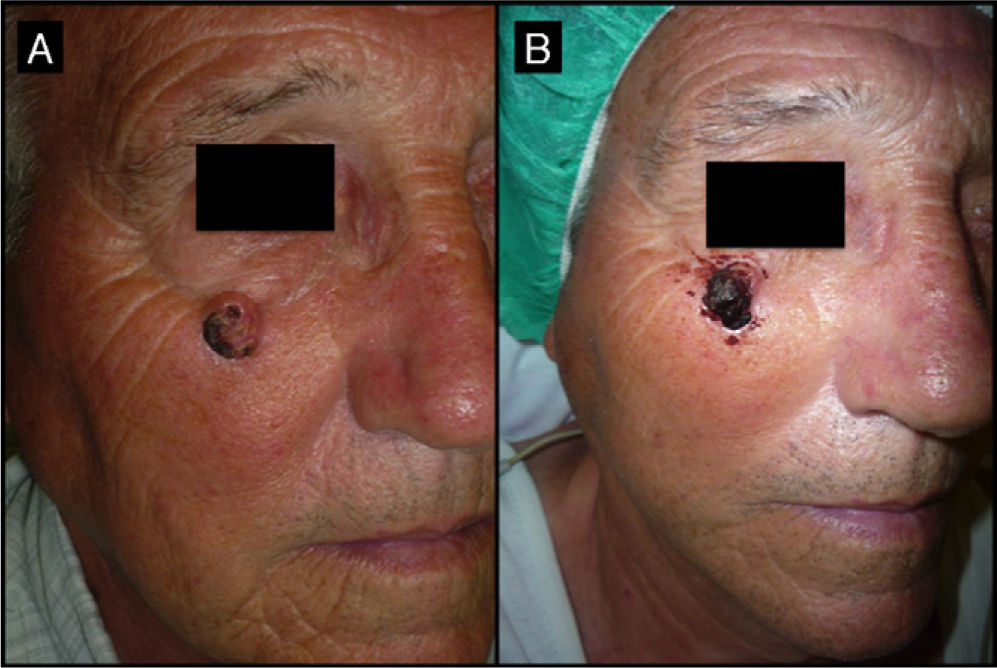
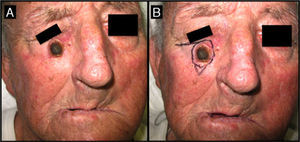
| 6/71/M (Fig. 6) | 2 | Right cheek | 2.3 | 2.4 | + 0.1 (5%) | 2.8 | Flap | Yes (2) |
| 7/70/M | 2 | Back of left hand | 1.8 | 2 | + 0.2 (10%) | 2.6 | Direct | No |
| 8/78/F | 3 | Left temple | 2.2 | 2.4 | + 0.2 (10%) | 2.9 | Flap | Yes (2) |
| 9/75/F | 2 | Right cheek | 2.5 | 2.5 | 0 (0%) | 3 | Flap | Yes (2) |
| 10/70/M | 1 | Left cheek | 2 | 2 | 0 (0%) | 2.4 | Direct | No |
| 11/71/M | 2 | Back of left hand | 1.8 | 2 | + 0.2 (10%) | 2.5 | Direct | No |
| 12/68/M | 3 | Right temple | 1.8 | 2 | + 0.2 (10%) | 2.5 | Direct | No |
| 13/59/F | 3 | Right bridge of nose | 1.7 | 2 | + 0.3 (+15%) | 2.4 | Direct | No |
| 14/65/F | 2 | Back of right hand | 1.5 | 1.5 | 0 (0%) | 2 | Direct | No |
| 15/67/F | 4 | Right temple | 1.5 | 1.4 | -0.1 (-10%) | 2 | Direct | Yes (1) |
Abbreviations: F, female; M, male.
The methotrexate group (Table 1) consisted of 5 men and 5 women with a mean age of 72 years. The mean time since appearance of the tumor in this group was 2.3 months. The lesion was located in the facial region in 7 patients (temple [n=4], periocular region [n=2], and bridge of the nose [n=1]) and in the acral region (back of the hand) in 3 patients (Figs. 1, 3–5). The median tumor size was 2cm (range, 1.5-2.5cm). The median volume of intralesional methotrexate required for the tumors to acquire a yellow color was 0.5mL (range, 0.3-0.5mL). No complications were observed during the administration of the drug.
Seventy-year-old man with keratoacanthoma in the right infrapalpebral region; the tumor had appeared 4 months earlier (patient 2, Table 1). A, Tumor with a diameter of 2cm at the baseline visit. B, Tumor, now measuring 1cm, a month after neoadjuvant treatment with intralesional methotrexate.
Seventy-two-year-old man with keratoacanthoma on the back of his left hand (patient 8, Table 1). A, Tumor measuring 2cm before intralesional injection of methotrexate. B, Tumor 1 month after treatment (65% reduction in size).
Fifty-two-old man with keratoacanthoma of 3 months’ duration under the right eyebrow (patient 3, Table 1). A, Tumor of 1.5cm at the baseline visit. B, Surgical excision of a tumor of this size would have required cutting the eyebrow. C, Tumor, now a small papule measuring 0.5cm, 1 month after treatment with intralesional methotrexate. The use of a small elliptical excision allowed the tumor to be removed without cutting the eyebrow, thereby achieving very good functional and cosmetic outcomes.
The surgery-only group (Table 2) consisted of 8 men and 7 women with a mean age of 70 years (range, 58-78 years) (Fig. 6). The median time since appearance of the tumor was 2 months (range, 1-4 months). The lesion was located in the facial region in 11 patients (cheek [n=5], temple [n=4], forehead [n=1], and bridge of the nose [n=1]) and in the acral region (back of the hand) in 4 patients. The median tumor size in this group was 2cm (range, 1.5-2.7cm).
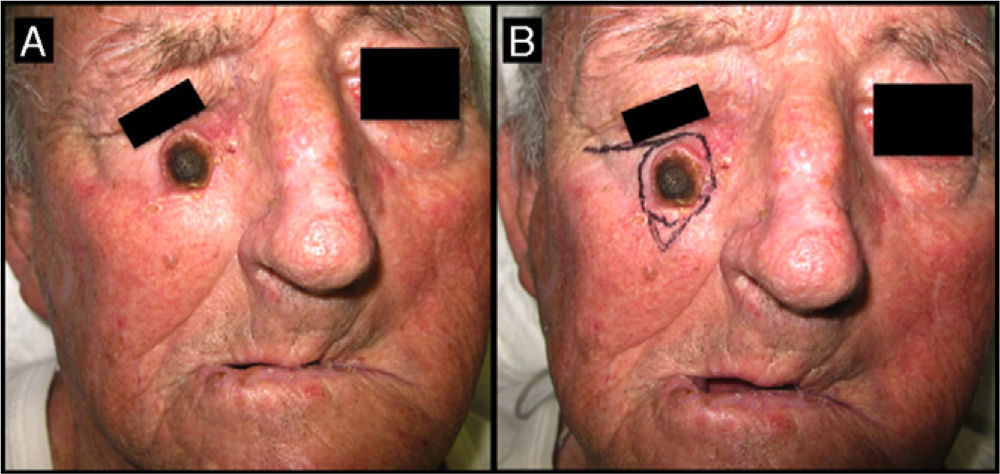
Seventy-one-year-old man with keratoacanthoma on his right cheek (patient 6, Table 2). A, Tumor with a diameter of 2.3cm at the baseline visit. B, Tumor measuring 2.8cm (not treated with intralesional methotrexate) at the time of surgery, 1 month later. An advancement flap was required to repair the surgical defect.
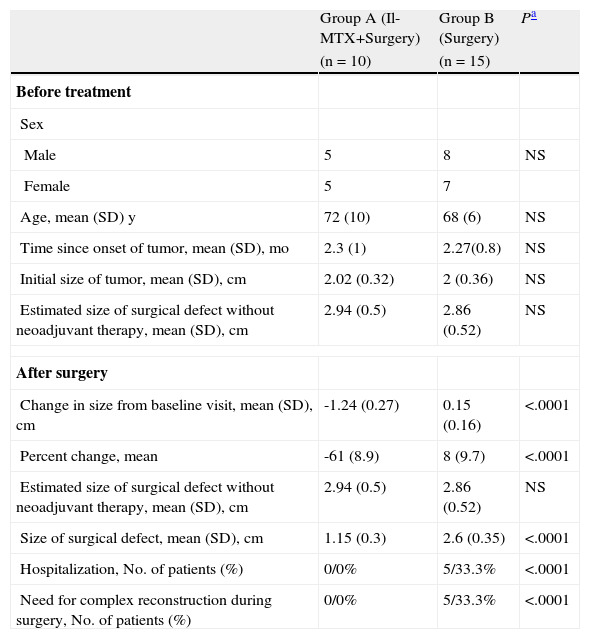
The results of the between-group comparisons are shown in Table 3. No significant differences were detected in age, time since onset of tumor, or size of the tumor at the baseline visit. Tumor size, however, was significantly smaller in the methotrexate group than in the surgery-only group at the time of surgery (difference of 1.3cm; 95% confidence interval [95% CI], 1.1-1.6cm). The decrease in tumor size was 69% greater (95% CI, 62%-77%) in the methotrexate group than in the surgery-only group. The size of the surgical defect was also smaller in this group (mean of 1.45cm less; 95% CI, 1.18-1.72cm).
Comparison of Groups A and B Before Treatment and After Surgery.
| Group A (Il-MTX+Surgery) | Group B (Surgery) | Pa | |
| (n=10) | (n=15) | ||
| Before treatment | |||
| Sex | |||
| Male | 5 | 8 | NS |
| Female | 5 | 7 | |
| Age, mean (SD) y | 72 (10) | 68 (6) | NS |
| Time since onset of tumor, mean (SD), mo | 2.3 (1) | 2.27(0.8) | NS |
| Initial size of tumor, mean (SD), cm | 2.02 (0.32) | 2 (0.36) | NS |
| Estimated size of surgical defect without neoadjuvant therapy, mean (SD), cm | 2.94 (0.5) | 2.86 (0.52) | NS |
| After surgery | |||
| Change in size from baseline visit, mean (SD), cm | -1.24 (0.27) | 0.15 (0.16) | <.0001 |
| Percent change, mean | -61 (8.9) | 8 (9.7) | <.0001 |
| Estimated size of surgical defect without neoadjuvant therapy, mean (SD), cm | 2.94 (0.5) | 2.86 (0.52) | NS |
| Size of surgical defect, mean (SD), cm | 1.15 (0.3) | 2.6 (0.35) | <.0001 |
| Hospitalization, No. of patients (%) | 0/0% | 5/33.3% | <.0001 |
| Need for complex reconstruction during surgery, No. of patients (%) | 0/0% | 5/33.3% | <.0001 |
Abbreviations: Il-MTX, intralesional methotrexate; NS, not significant.
In a post-hoc analysis comparing the difference between the real size of the surgical defect and the estimated size (calculated using the initial tumor size and recommended surgical margins for cutaneous squamous cell carcinoma [4mm for tumors < 2cm and 6mm for tumors > 2cm]27, it was seen that the actual size was 1.53cm smaller (95% CI, 1.26-1.8cm) in the methotrexate group than in the surgery-only group.
All the surgical defects in the methotrexate group were repaired using the direct closure technique. Furthermore, none of the patients in this group required hospitalization, experienced clinical complications, or had abnormal laboratory test results due to the administration of intralesional methotrexate. In the surgery-only group, direct closure was possible in 10 patients and a skin flap was necessary in the other 5 patients. Five patients in this group also required hospitalization for 1 or 2 days.
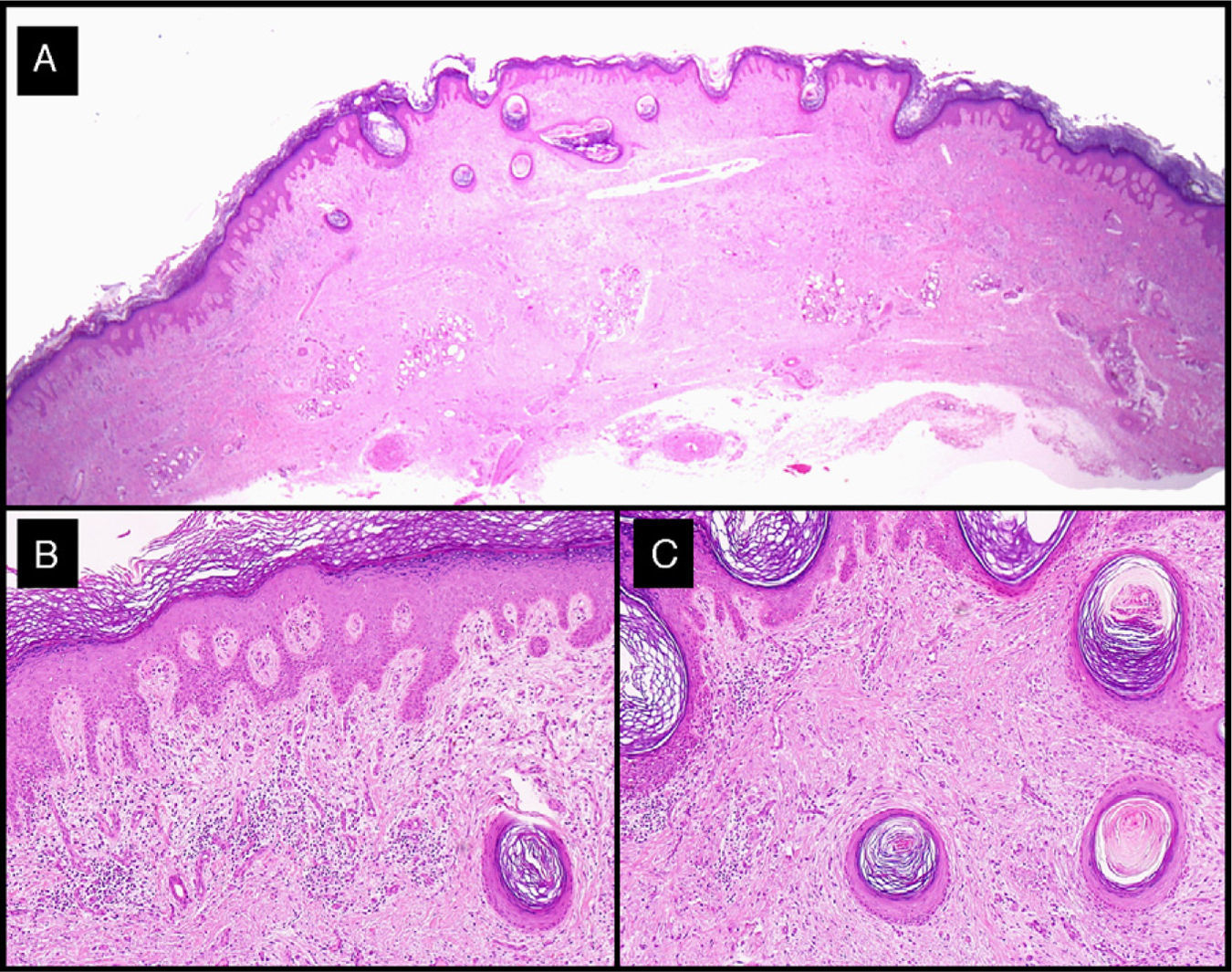
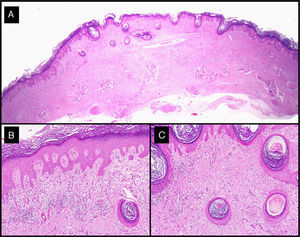
Histologic analysis of the surgical specimens showed typical features of keratoacanthoma. Of note in tumors treated with intralesional methotrexate was a dense lymphocytic inflammatory infiltrate with a variable number of foreign body giant cells (Fig. 7).
Histologic features of excised residual lesion after the administration of intralesional methotrexate (patient 3, Table 1). A-C, Dysplastic epidermis in association with a dense lymphohistiocytic infiltrate and some foreign body giant cells corresponding to the regression of the tumor following the administration of intralesional methotrexate (hematoxylin-eosin staining; original magnification: A, ×10; B, ×40; C, ×100).
Keratoacanthoma, which is also known as molluscum sebaceum, is a fast-growing skin tumor—possibly originating from the infundibulum of the hair follicle—that develops on sun-exposed skin.1,2 Its classification has been a subject of debate as it has been described in the literature as a benign tumor, a pseudomalignant tumor, a malignant self-involuting tumor, and a subtype of squamous cell carcinoma.1,2,28–35 These differences in proposed classification are explained by the fact that the clinical course of keratoacanthoma is both similar to and different from that of the variants of squamous cell carcinoma that have been described, with extremely rapid growth followed, in some cases, by partial regression.
Occasionally, keratoacanthoma can undergo complete spontaneous regression within a variable time period of a few months (generally 8 weeks).36,37 Although there have been few cases reported, keratoacanthoma can also exhibit malignant behavior, with perineural invasion and even distant metastasis.28–35 According to Hodak et al,29 this justifies classifying this tumor as a squamous cell carcinoma with low metastatic potential. Sánchez Yus et al37 and Weedon et al38 consider that keratoacanthoma is similar to Bowen's disease in this respect, that is, it is a premalignant tumor with a risk of degenerating into squamous cell carcinoma in up to 25% of cases. Mandrell et al,36 in contrast, consider keratoacanthoma to be a benign self-involuting tumor, and stated that the cases of metastasizing keratoacanthomas reported were probably diagnostic errors and were really squamous cell carcinomas
In our opinion, keratoacanthoma is a well-differentiated squamous cell carcinoma with low metastatic potential. The fact that most of the tumors in the surgery-only group in our study did not return seems to support this hypothesis. Indeed, 10 (66%) of the 15 tumors in this group increased in size in the 30 to 35 days between the baseline visit and surgery; the increase in size ranged from 5% to 20%. Four of the tumors remained stable and just 1 became smaller (reduction of 10%).
The treatment of choice for keratoacanthoma is complete surgical excision. This is for 2 reasons: first, in order to establish a definitive diagnosis, it is necessary to analyze the entire lesion to rule out foci of dermal invasion,36 and second, if we assume that keratoacanthoma is a malignant subtype of squamous cell carcinoma, the most appropriate treatment according to the recently published National Comprehensive Cancer Network treatment guidelines is surgical excision with safety margins of 4mm for tumors with a diameter of less than 2cm and of 6mm for larger tumors.37
The surgical removal of keratoacanthoma is associated with considerable morbidity, particularly in the case of large tumors (diameter of over 1.5cm) and tumors located in the facial region (due to the risk of cosmetic and/or functional impairment) or on the back of the hands or feet (due to the risk of considerable functional impairment) (Fig. 5). In such cases, it may also be necessary to use flaps and/or grafts, leading to considerably lengthened surgery times and an increased risk of complications.
We have shown that the administration of neoadjuvant intralesional methotrexate in patients scheduled for surgical excision of keratoacanthoma and without contraindications for methotrexate can significantly decrease tumor size without interfering with routine dermatology practice. In our study, a single injection of methotrexate 30 days before the operation led to a 50% to 80% reduction in tumor size (Figs. 1, 3–5). By contrast, only 1 of the tumors in the surgery-only group decreased in size, and the reduction was only slight; the rest stayed the same size or grew (Fig. 6). Intralesional methotrexate also led to a significant reduction in the size of the surgical defect (mean decrease of 1.45cm). A post-hoc analysis based on estimated surgical defect sizes indicated that treatment with intralesional methotrexate reduced the size of the surgical defect by a mean of 1.7cm. Finally, the neoadjuvant treatment also simplified surgery as all of the surgical defects in this group were repaired using direct closure. In the other group, 5 (30%) of the patients required a skin flap.One alternative to the use of intralesional methotrexate as neoadjuvant therapy in keratoacanthoma is 5-fluorouracil. We opted against this, however, for several reasons. 5-Fluorouracil requires local anesthetic as its injection causes intense pain. Furthermore, it needs to be administered weekly to achieve the desired effect (hence more visits to the dermatologist) and it causes necrosis of the tumor, complicating subsequent excision.11–14,24 By contrast, based on the observations of our study, intralesional methotrexate can be administered in a single injection without local anesthetic and without causing necrosis, meaning its use will not increase the number of visits to the dermatologist or the waiting times for surgery (generally between 26 and 60 days).39 The only disadvantage of intralesional methotrexate compared to 5-fluorouracil is that laboratory tests are required to rule out blood, liver, or kidney disorders.15–24
While the current study has yielded interesting conclusions, it has several limitations. First, the fact that the treatment was not blinded might have led to a certain bias in that the results might have been interpreted as being more positive than they really were. To control for this, changes in tumor size and appearance were analyzed, using photographs, by observers not involved in the study. Second, a large proportion of patients diagnosed with keratoacanthoma were excluded from the study. This occurred because our intention was to include only patients who would truly benefit from the neoadjuvant therapy. This, combined with the fact that keratoacanthoma is not widely seen in routine clinical practice, means that relevant conclusions can be drawn from the results obtained. Nonetheless, it should not be forgotten that because of the small sample size, the study lacked statistical power to detect effects related to our secondary objectives, particularly adverse effects due to intralesional methotrexate. It should be noted, however, that the absence of adverse effects due to methotrexate in this study is consistent with data reported in the literature.24 With respect to the risk of systemic effects of methotrexate, it should be noted that the maximum doses used in the current study correspond to systemic doses that are commonly administered in many diseases, including psoriasis in particular. Furthermore, intralesional methotrexate has been used, without complications, on previous occasions.
To conclude, given the simplicity of the technique and the lack of adverse events associated with its use in our study, the use of intralesional methotrexate should be considered prior to the surgical excision of keratoacanthomas with a diameter of over 1.5cm, particularly in the case of tumors located in the facial and acral regions.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Martorell-Calatayud A, et al. Ensayo clínico: la infiltración intralesional con metotrexato de forma neoadyuvante en la cirugía del queratoacantoma permite obtener mejores resultados estéticos y funcionales. Actas Dermosifiliogr.2011;102:605-15.