Alitretinoin (9-cis-retinoic acid) is an endogenous derivative of vitamin A and functions as an agonist of both families of nuclear receptors (retinoic acid receptor-α, -β, -γ; retinoid X receptor-α, -β, -γ). It has been investigated in the treatment of chronic hand eczema in many studies in recent years and the results have been promising.
ObjectivesTo evaluate the efficacy and safety of oral alitretinoin in the treatment of chronic hand eczema that is refractory to treatment with potent topical corticosteroids and to analyze the long-term response to treatment.
Materials and methodsA prospective, observational, descriptive study was undertaken in 15 patients with chronic hand eczema that was refractory to treatment with potent topical corticosteroids. Patients were administered oral alitretinoin 30mg/d for 3 months followed by 6 months of follow-up.
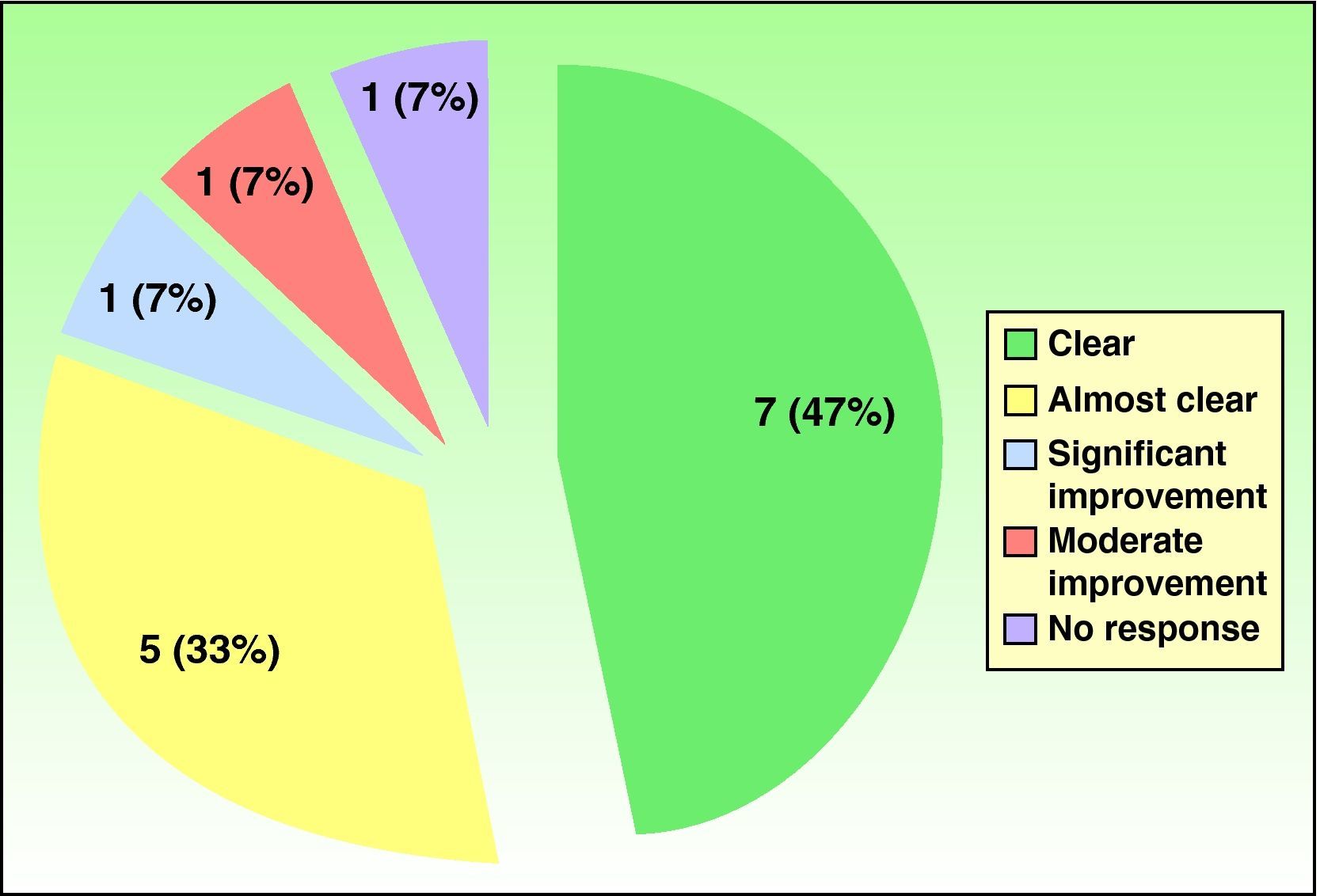
ResultsA complete response, with “clear” hands was obtained in 7 patients (47%), 5 patients (33%) achieved a partial response (almost clear hands), 1 patient (7%) showed substantial improvement, 1 (7%) showed moderate improvement, and 1 patient (7%) did not respond to treatment. Relapse occurred within 6 months of treatment suspension in 54% of cases. The treatment was well tolerated. Side effects, observed in 50% of cases, were mild (headache, elevated lipid levels, slightly elevated transaminase levels, and epigastric pain), except in 1 patient, who had a substantial reduction in thyroid stimulating hormone levels.
ConclusionsThe results of our study support the proposal of alitretinoin as an effective and safe short-term and medium-term treatment for chronic hand eczema in patients whose disease is refractory to treatment with potent topical corticosteroids.
alitretinoína (ácido 9-cis-retinoico) es un derivado endógeno de la vitamina A, panagonista de ambas familias de receptores nucleares (RAR-α, -β, -γ, RXR-α, -β, -γ) estudiado en el eczema crónico de manos en diversos trabajos durante los últimos años con resultados prometedores.
Objetivosevaluar la eficacia y seguridad de alitretinoína oral en el tratamiento del eczema crónico de manos refractario al tratamiento con corticoides tópicos potentes, y su respuesta mantenida a largo plazo.
Material y métodosllevamos a cabo un estudio prospectivo, descriptivo y observacional. Se reclutaron un total de 15 pacientes con eczema crónico de manos refractarios al tratamiento con corticoides tópicos potentes, a los que se les administró una dosis de alitretinoína oral de 30mg/día durante tres meses, con un seguimiento posterior de 6 meses.
Resultadosun total de 7 pacientes (47%) obtuvo respuesta completa con «manos limpias» al finalizar el tratamiento, 5 pacientes (33%) alcanzaron una respuesta parcial «manos casi limpias», un paciente (7%) obtuvo mejoría importante, otro paciente (7%) evidenció mejoría moderada y sólo un paciente (7%) no respondió al tratamiento. El porcentaje de recaída durante los 6 meses después de haber terminado el tratamiento fue del 54%. El tratamiento fue bien tolerado con efectos colaterales en el 50% de los pacientes, considerados leves (cefalea, hiperlipidemias, aumento leve de transaminasas, epigastralgias), excepto un paciente, que presentó una disminución importante de la hormona estimulante del tiroides.
Conclusioneslos resultados presentados son coherentes con la propuesta de alitretinoína como un medicamento eficaz y seguro para el tratamiento a corto y medio plazo del eczema crónico de manos en pacientes refractarios a tratamiento con corticoides tópicos potentes.
Chronic eczema, which is the most common skin disease affecting the hands, has been found to vary in prevalence from year to year. Estimates range from 7% to 12% in general populations in northern European countries and may be higher in the United States.1–3 Hand eczema occurs more frequently in women (ratio of women to men, 2:1) and in young adults (aged 20-50 years), who account for most of the economically active population. This condition therefore has considerable impact in the workplace and has been estimated to account for between 10% and 25% of occupational illness,4 although the real prevalence may be 30-to-50-fold higher given that not all cases are reported.5
Available treatments are wide ranging, but as a rule their effectiveness is limited. Currently, hand eczema is managed by means of lifestyle changes in the first instance, by avoiding allergens found to be responsible for the condition, and also by means of various topical medications. Topical corticosteroids6 are the first line of treatment. Alternatives include calcineurin inhibitors,7 bexarotene gel,8 botulinum toxin,9, and targeted phototherapy.10
When topical treatment fails, oral systemic corticosteroids (ciclosporin, methotrexate, retinoids, azathioprine, mycophenolate mofetil, and even infliximab) may be prescribed.11–14 Systemic drugs are inappropriate for managing chronic conditions, however, given the considerable level of systemic toxicity and the frequency of flare-ups when treatment is withdrawn. New therapeutic options for hand eczema are therefore warranted.
According to the records of occupational accident and health insurers in Spain, flare-ups of hand eczema account for 97.8% of medically related leave of absence from work and the annual cost per patient with this condition comes to €42000.15
The European Dermato-Epidemiology Network found that of 90 studies on treatments for chronic hand eczema published between 1977 and 2003, only 31 were randomized controlled trials and only around 1200 patients had been enrolled in all.16 At that point, few therapeutic options were available for long-term use in patients with severe chronic hand eczema refractory to potent topical corticosteroids. Recent studies of alitretinoin (9-cis-retinoic acid), an endogenous derivative of vitamin A that functions as an agonist of both families of nuclear receptors (retinoic acid receptors α and β and retinoid X receptors α, β, and γ) point to this drug as the treatment of choice for chronic hand eczema resistant to other measures. The mechanism of action of alitretinoin is unknown, but immunomodulatory and anti-inflammatory effects, together with effects on angiogenesis and keratinization, are probably involved.17–19
ObjectivesThis study aimed to assess the safety and efficacy of oral alitretinoin in patients with chronic hand eczema refractory to potent topical corticosteroids, to analyze the long-term response to treatment, and to compare outcomes in our patients with the results reported in the literature.
Material and MethodsWe performed a prospective descriptive open-label observational study in patients with chronic hand eczema that was refractory to treatment with potent topical corticosteroids. The patients were under care at the eczema unit of Hospital Ramón y Cajal. The inclusion criteria were as follows:
- 1.
Age between 18 and 75 years
- 2.
Correct clinical diagnosis of chronic hand eczema from any cause
- 3.
Eczema refractory to topical corticosteroid treatment applied for at least 6 months
- 4.
Patients’ provision of signed, informed consent to inclusion in the study
The exclusion criteria were as follows:
- 1.
Bacterial, fungal, or viral infection
- 2.
Other disease under treatment with systemic medications that might interfere with the interpretation of outcomes
- 3.
History of use of phototherapy, oral corticosteroids, or immunosuppressants within 2 months of enrollment
- 4.
Transaminase concentration > 250% over the upper limit of normal, triglyceride concentration > 200% over the upper limit of normal, total cholesterol > 200% over the upper limit of normal
All patients underwent patch testing with the standard panel of the Spanish Contact Dermatitis and Skin Allergy Research Group (GEIDAC). Blood tests (including biochemistry, complete blood count, indicators of thyroid function, metabolic markers, and liver enzymes) were also performed. Women of childbearing age had a pregnancy test before treatment and 5 months after the final treatment. Digital photographs of the hands were taken at baseline, during treatment, and at the end of treatment. Oral alitretinoin (30mg) was taken daily. Weekly check-ups at first and later monthly check-ups (including blood tests) were scheduled until the end of 3 months of treatment. Dosage reductions were not allowed, but treatment was interrupted in case of a severe adverse event. Patients continued to use only plain moisturizing lotions.
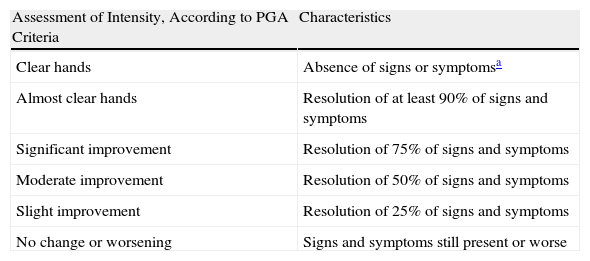
The clinical response to treatment was assessed by means of the Physician's Global Assessment (PGA) scale (Table 1) based on improvement in signs and reported symptoms (erythema, scaling, hyperkeratosis, vesicles, edema, fissures, pruritus, or pain) at the moment of enrollment, at 1 week, and monthly during treatment. A patient with clear or almost clear hands was considered to have responded. Included were those with a mean reduction of up to 75% of the signs and symptoms at the onset of disease. At the end of treatment the patient was followed for 6 months to check for relapse (recurrence of 75% of the initial signs and symptoms). Adverse effects of treatment were also recorded.
Measures of Efficacy: Physician's Global Assessment (PGA).
| Assessment of Intensity, According to PGA Criteria | Characteristics |
| Clear hands | Absence of signs or symptomsa |
| Almost clear hands | Resolution of at least 90% of signs and symptoms |
| Significant improvement | Resolution of 75% of signs and symptoms |
| Moderate improvement | Resolution of 50% of signs and symptoms |
| Slight improvement | Resolution of 25% of signs and symptoms |
| No change or worsening | Signs and symptoms still present or worse |
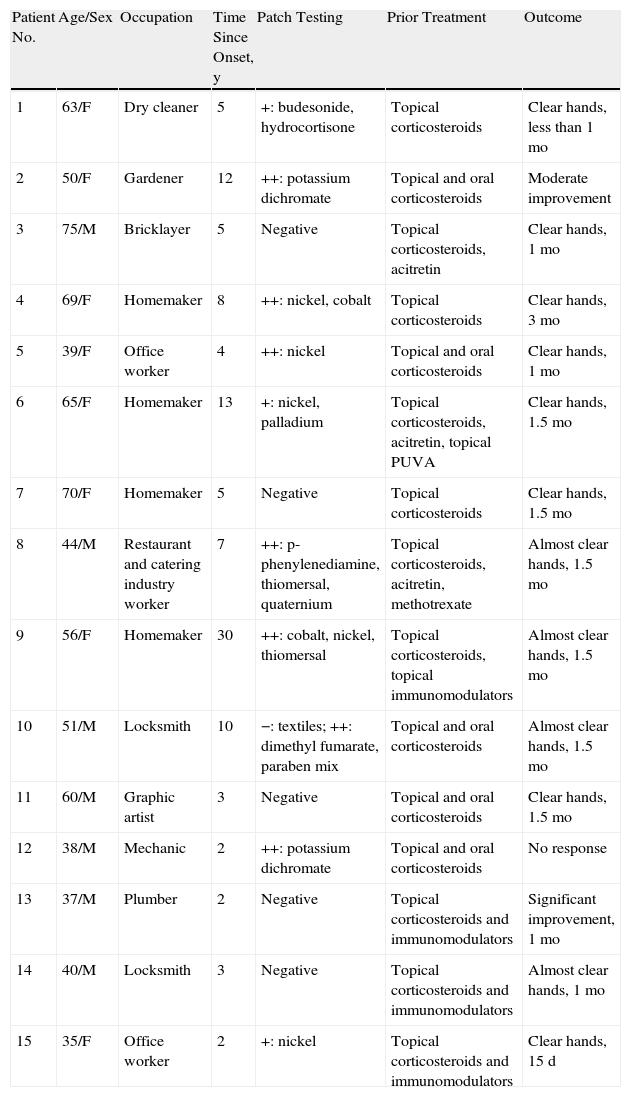
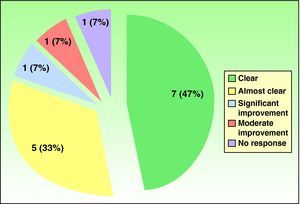
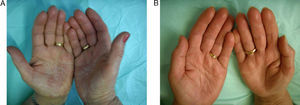
Fifteen patients (7 men, 8 women) were treated (Table 2). The mean age was 53 years (range, 35-75 years). The mean duration of disease since onset of lesions before treatment was 7.4 years. Patch tests were positive in 67% of the patients, although the results were considered relevant to hand eczema in only 14%. Most of the cases in this patient series, which included slightly more women, were of irritative eczema. Half the patients had required systemic treatment previously (oral corticosteroids, phototherapy, acitretin, methotrexate) because of lack of response to topical corticosteroids. Seven patients (47%) had a complete response (clear hands) at the end of treatment, 5 (33%) had almost clear hands, 1 (7%) had significant improvement, 1 (7%) had moderate improvement, and 1 (7%) had no response (Figure 1). For 92% of the patients, response was evident before 2 months (range, 15 days– 3 months). The rate of relapse (recurrence of 75% of the signs and symptoms at the onset of disease) was 54% during the 6 months after alitretinoin treatment ended. Alitretinoin was well tolerated. Although half the patients experienced adverse events, these effects were slight and did not require suspension of treatment or additional medication.
Summary of Patient Characteristics and Outcomes.
| Patient No. | Age/Sex | Occupation | Time Since Onset, y | Patch Testing | Prior Treatment | Outcome |
| 1 | 63/F | Dry cleaner | 5 | +: budesonide, hydrocortisone | Topical corticosteroids | Clear hands, less than 1 mo |
| 2 | 50/F | Gardener | 12 | ++: potassium dichromate | Topical and oral corticosteroids | Moderate improvement |
| 3 | 75/M | Bricklayer | 5 | Negative | Topical corticosteroids, acitretin | Clear hands, 1 mo |
| 4 | 69/F | Homemaker | 8 | ++: nickel, cobalt | Topical corticosteroids | Clear hands, 3 mo |
| 5 | 39/F | Office worker | 4 | ++: nickel | Topical and oral corticosteroids | Clear hands, 1 mo |
| 6 | 65/F | Homemaker | 13 | +: nickel, palladium | Topical corticosteroids, acitretin, topical PUVA | Clear hands, 1.5 mo |
| 7 | 70/F | Homemaker | 5 | Negative | Topical corticosteroids | Clear hands, 1.5 mo |
| 8 | 44/M | Restaurant and catering industry worker | 7 | ++: p-phenylenediamine, thiomersal, quaternium | Topical corticosteroids, acitretin, methotrexate | Almost clear hands, 1.5 mo |
| 9 | 56/F | Homemaker | 30 | ++: cobalt, nickel, thiomersal | Topical corticosteroids, topical immunomodulators | Almost clear hands, 1.5 mo |
| 10 | 51/M | Locksmith | 10 | −: textiles; ++: dimethyl fumarate, paraben mix | Topical and oral corticosteroids | Almost clear hands, 1.5 mo |
| 11 | 60/M | Graphic artist | 3 | Negative | Topical and oral corticosteroids | Clear hands, 1.5 mo |
| 12 | 38/M | Mechanic | 2 | ++: potassium dichromate | Topical and oral corticosteroids | No response |
| 13 | 37/M | Plumber | 2 | Negative | Topical corticosteroids and immunomodulators | Significant improvement, 1 mo |
| 14 | 40/M | Locksmith | 3 | Negative | Topical corticosteroids and immunomodulators | Almost clear hands, 1 mo |
| 15 | 35/F | Office worker | 2 | +: nickel | Topical corticosteroids and immunomodulators | Clear hands, 15 d |
Abbreviations: F, female; M, male; PUVA, psoralen UVA.
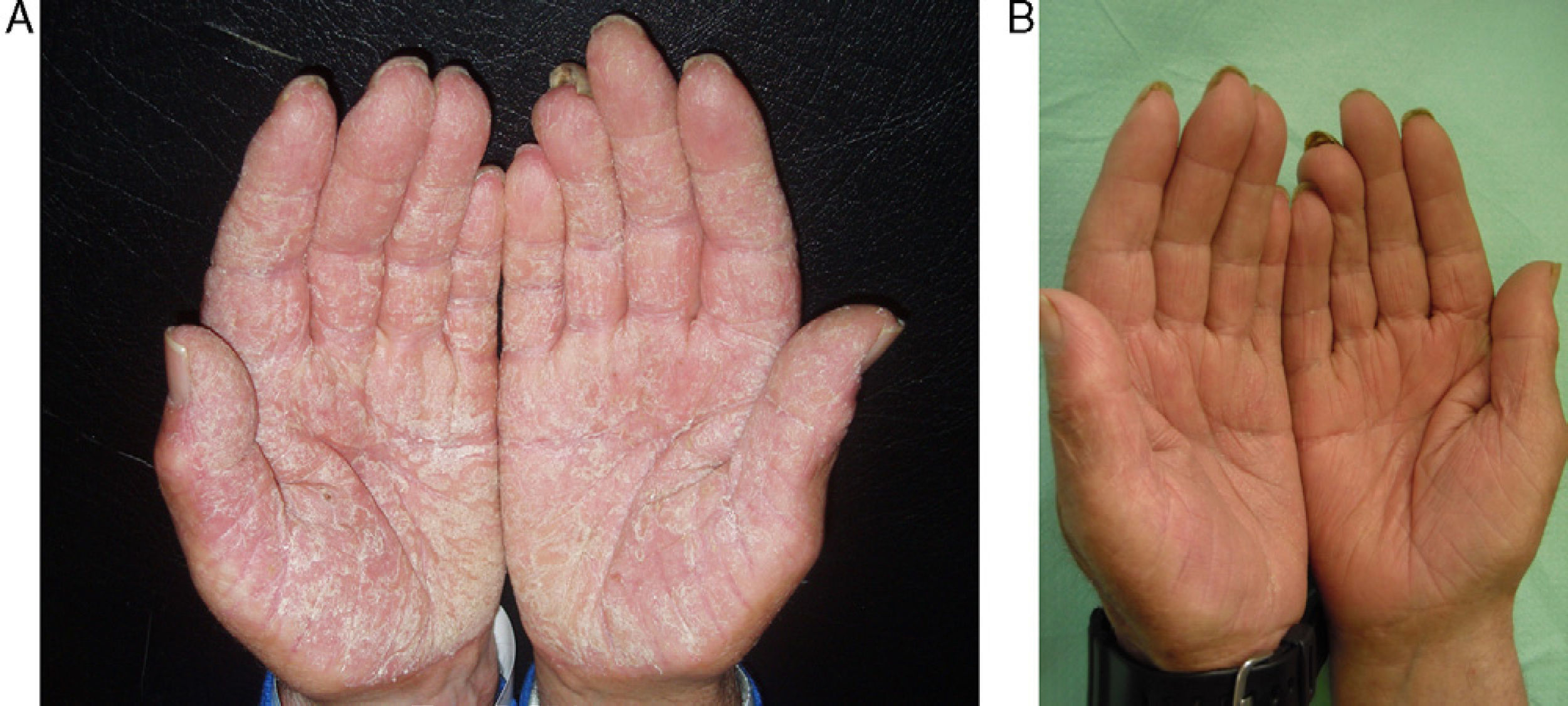
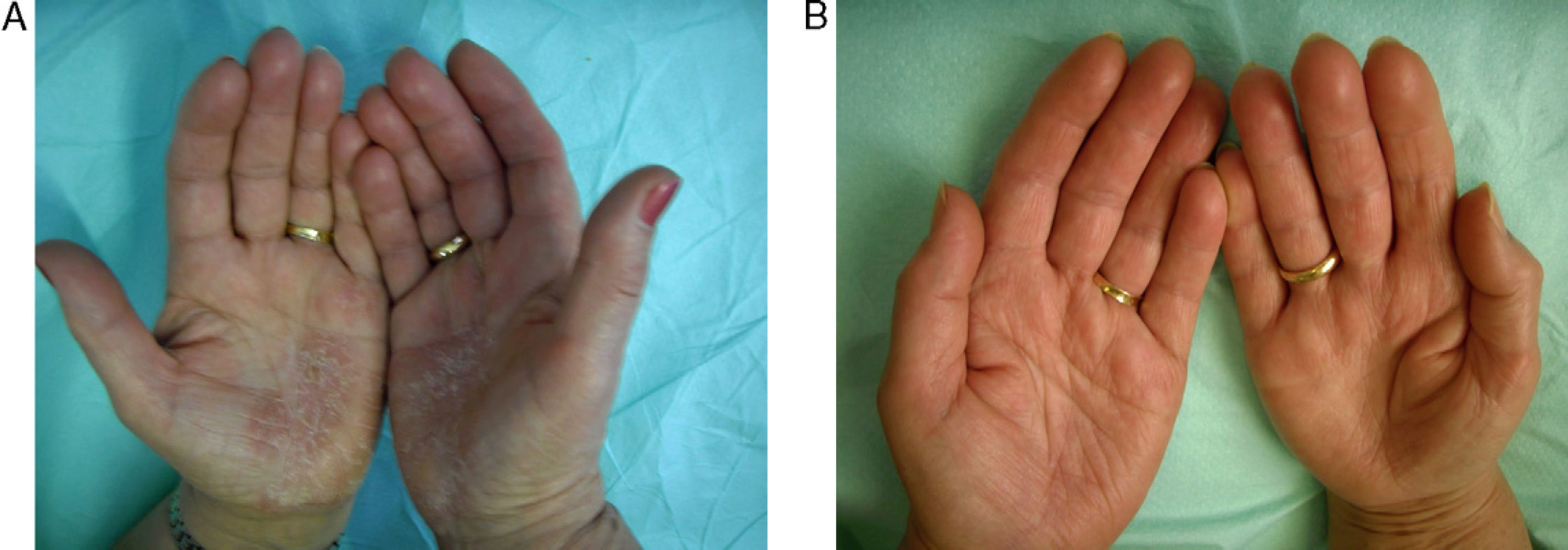
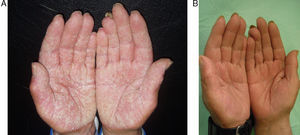
The most frequent adverse effects were slight increases in cholesterol (40%), headache (13%), raised transaminase levels (7%) and epigastric pain (7%). In 1 case (7%) there was a significant decrease in thyroid stimulating hormone. Results are summarized in Table 3. Figures 2 and 3 show examples of a very good response to oral alitretinoin.
Since 1999 a total of 1635 patients with chronic hand eczema and 364 healthy volunteers have been treated with alitretinoin in clinical trials, with good results18,19; the results observed in our patient series were similar.
Alitretinoin treatment led to satisfactory outcomes in this study, in which 87% of patients had an average reduction of 75% of signs and symptoms at onset (good response) and 47% (7 patients) had clear hands at the end of treatment. These rates are consistent with results from other small series reports, although the outcomes of large controlled trials have been less positive.17–19 Differences in the good-response rates reported can be attributed to the size of the series; ours was similar to that of Bollag and Ott.17
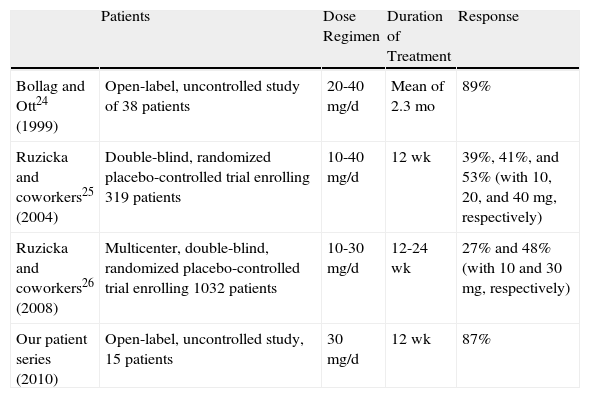
Table 4 summarizes the literature and the findings we report in this paper. Response rates have ranged from 27% to 89%, according to dosage and duration of therapy. In our series, response failure occurred in 7% (a single patient), a figure that is similar to the 5.5% failure rate in the report of Bollag and Ott.17
Comparison Between Our Results and Outcomes From the Main Clinical Trials on Oral Alitretinoin for Chronic Hand Eczema Refractory to Topical Corticosteroids.
| Patients | Dose Regimen | Duration of Treatment | Response | |
| Bollag and Ott24 (1999) | Open-label, uncontrolled study of 38 patients | 20-40 mg/d | Mean of 2.3 mo | 89% |
| Ruzicka and coworkers25 (2004) | Double-blind, randomized placebo-controlled trial enrolling 319 patients | 10-40 mg/d | 12 wk | 39%, 41%, and 53% (with 10, 20, and 40mg, respectively) |
| Ruzicka and coworkers26 (2008) | Multicenter, double-blind, randomized placebo-controlled trial enrolling 1032 patients | 10-30 mg/d | 12-24 wk | 27% and 48% (with 10 and 30mg, respectively) |
| Our patient series (2010) | Open-label, uncontrolled study, 15 patients | 30 mg/d | 12 wk | 87% |
Regarding mean duration of treatment until response, clearing was remarkably rapid in our study. We observed a response within 40 days, nearly half the time reported previously.19
The adverse effects (headache, hyperlipidemia, epigastric pain, and elevated transaminase concentrations) were similar to those seen in earlier studies. Specific treatment was not required when these conditions occurred. Only 1 patient had a significant reduction in thyroid stimulating hormone level, which had returned to normal at follow-up testing without a decrease in the alitretinoin dose. No mucocutaneous changes were observed during therapy. Treatment was never stopped in our series, although in previous trials patients were withdrawn because of severe headache.18,19
Regarding long-term efficacy, 46% of our patients did not experience a relapse in 6 months of follow-up. That rate is somewhat lower than previous reports, in which 65% of patients maintained good response on 6-month follow-up.19 Interestingly, the 2 patients in our series who achieved clear hands during treatment experienced an exacerbation within 3 months of treatment. The flare-up was attributed to seasonal changes but did not affect the overall good response in these cases.
A main limitation of our study, the small size, might explain the small differences in response rates, mean time elapsed until response, and long-term maintenance of effect between our study and larger ones. Our study also lacked a control group and we found no Spanish studies whose results we can compare with ours. Our observations are encouraging, however, in that alitretinoin was efficacious in the short-term and had an acceptable long-term effect after treatment ended.
It would also have been interesting to compare which type of eczema responds best to oral alitretinoin, given that several studies report superior results when hyperkeratosis is present. We studied too few patients to be able to make that comparison.
One recent study reported that an alitretinoin retreatment cycle (30mg/d) achieved a response in 80% of the patients and that tolerance was good.20 In fact our group now has experience with 3 patients who responded well to alitretinoin in a first cycle and who also responded to retreatment after a relapse.
Finally, a Swiss study comparing the cost-effectiveness of alitretinoin to other measures of support for patients with refractory hand eczema found that response rates were 3-fold higher in alitretinoin-treated patients.21 The authors concluded that use of this drug is cost-effective.
In July 2008, the European Medicines Agency (EMA) approved the sale of oral alitretinoin in several European countries for cases of severe chronic hand eczema that is unresponsive to potent topical corticosteroids.22,23 The Spanish Agency for Medicines and Health Care Products (AEMPS) approved this drug in Spain only for compassionate use for the same indication; prescription began in 2009.
ConclusionsOur experience, which is consistent with the recent literature on oral alitretinoin, suggests that this drug is safe and efficacious in patients with chronic hand eczema that is refractory to potent topical corticosteroids. Alitretinoin is well tolerated, onset of action is rapid, and adverse effects are few. This drug should be considered useful in these patients. However, most patients experience a relapse within 6 months of stopping treatment, obliging the physician to consider prescribing retreatment protocols which are not yet fully established.
We hope that these findings can serve as the basis for larger studies in Spain, including cost-effectiveness studies that would favor making this drug more widely available to our patients.
Conflict of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Aguayo-Leiva IR, et al. Respuesta al tratamiento con alitretinoína oral en ECM refractario con eczema crónico de manos refractario al tratamiento con corticoides tópicos potentes: nuestra experiencia en 15 pacientes. Actas Dermosifiliogr. 2011;102:616-622.