Quality management systems (QMS) are tools that serve to structure, control and improve the usual activities that take place in an organization or service.
The ISO 9001:2015 is an internationally recognized standard, which provides the necessary resources to help an organization to improve its performance, based on the principle of plan-do-control-act, in order to obtain continuous improvement. In the field of health, it is an essential tool for the management of the services offered to patients.
The ISO quality certification allows to demonstrate compliance, according to established quality standards.
The process of implementing a QMS follows several phases that culminate with the completion of an external audit, which once passed, allows obtaining the quality certification ISO 9001:2015.
This article describes the steps to follow to obtain this certification in a Dermatology Service.
Los sistemas de gestión de calidad (SGC) son herramientas que sirven para estructurar, controlar y mejorar las actividades habituales que se desarrollan en una organización o servicio.
La norma ISO 9001:2015 es una norma reconocida internacionalmente que proporciona los recursos necesarios para ayudar a una organización a mejorar su rendimiento, basándose en el principio de planificar-hacer-controlar-actuar, con el fin de obtener una mejora continua. En sanidad es una herramienta clave para la gestión de los servicios ofrecidos a los pacientes.
La certificación de calidad ISO permite demostrar el cumplimiento de dicha norma, de acuerdo a unos estándares de calidad establecidos.
El proceso de implantación de un SGC siguiendo esta norma debe seguir varias fases, que culminan con la realización de una auditoría externa que, una vez superada, permite obtener la certificación de calidad ISO 9001:2015.
En este artículo se describen los pasos a seguir para obtener dicha certificación en un Servicio de Dermatología.
Within the health sector, both health professionals and patients require and demand a certain level of services. In the past, we have applied the premise that things are done properly, but this is no longer enough and it is necessary to demonstrate that a sufficient level of quality is offered. Quality Management Systems (QMS) provide the tools to evaluate how we are working and what our previsions are at any time.
This article discusses the topic of QMS and quality certification and explains the process to follow to create a QMS and obtain ISO certification, based on the experience of the dermatology department of the Hospital General Universitario in Alicante (HGUA), in Spain.
What is a Quality Management System and What is It Used for?QMS are tools that help structure, organize, control, and improve everyday activities performed within an organization or department, based on established requirements, which are documented, with the aim of influencing client satisfaction and improving the desired outcomes for the organization.1
Although QMS were first implemented in manufacturing, they can be applied to any sector, including health services. Terms such as quality, client satisfaction, process control, and certifications, frequently used in the business sector, are therefore now increasingly common in the health sector.2,3
Health professionals today are aware that patients and their families have high expectations for the services on offer and these professionals need tools to achieve excellence in the quality that they provide.
There are many definitions of quality. In general though, quality implies a set of properties inherent to a product that enables it to be characterized and assessed with respect to other similar products. In a management system, we would define quality as “the set of properties and characteristics that a service has to comply with to be able to satisfy the needs of the clients with minimal errors and defects.”4
In the context of the health sector, the concept is more difficult to define. According to the World Health Organization, the aim of quality assurance in health care is to ensure the patient receives the most appropriate the set of diagnostic and therapeutic services with a view to achieving optimal health care.5 Quality assurance should take into account all relevant factors and the extent of knowledge about the patient and about themedical service in order to achieve the best outcome with minimal risk of iatrogenic effects and maximum patient satisfaction with the process.5
What is Quality Certification?Quality certification is accreditation that indicates compliance with an existing standard for elaboration or execution of a product or service. It is distinct from a guarantee, safety, or prestige. Certification, according to the requirements of a standard, is the satisfactory outcome of an evaluation conducted by an independent third party. A standard is a document of voluntary application that contains technical specifications based on the results of experience and technological development and that guarantees compliance with levels of quality and safety. To assess the quality of health care, quantification is required through units of measurements that can be compared with previously established values and that are denoted benchmarks.
Although there are different accreditation systems (Joint Commission, EFQM),6–8 the standards of the ISO 9000 family are standards of a QMS of renowned international prestige that encompass several aspects of quality control.
There are other ISO standards, with quality specifications for more specific processes, such as environmental management systems (ISO 14001:2004), occupational health and safety management (OHSAS 18001), food safety management (ISO 22000), information technology management (ISO 27000,ISO 20000), health and social services, road traffic safety (ISO 39001), automation (ISO 16949), aerospace (ISO 9100), construction, energy management (ISO 50001), and tourism services (ISO 18513:2003). Almost all the seals that certify a company as ISO compliant are followed by a number. ISO stands for International Organization of Standardization, and the number indicates the type of product or service whose quality is certified. Sometimes, the letters ISO are preceded by the letters UNE EN, indicating a Spanish-European standard.
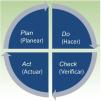
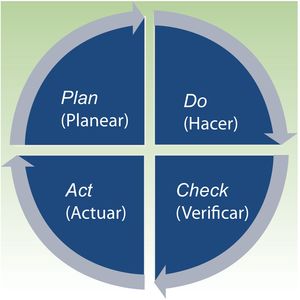
What is ISO 9001:2015?ISO 9001:2015 (the most recent version of ISO 9001) establishes the requirements to comply with a QMS and is applicable to any organization, regardless of size and geographic location. It covers the infrastructure, procedures, processes, and resources necessary to help organizations control and improve their performance and guide them towards efficiency, client service, and product excellence. QMS is based on the Deming cycle of plan, do, check, act (PDCA), to implement continuous improvement (Fig. 1).9–11
Health care is one of the sectors in which quality, training of personnel, technological development, and collective work capacity have an impact on health and patient care.9,12 ISO certification can be applied globally to institutions or health centers, whether public or private, or partially, to services or laboratories, and represents a seal of quality for users or patients who are served by them.
How is ISO Certification Obtained?To obtain a certificate, it is necessary to accredit before a certifying entity that the processes are being performed according to the ISO standard for which certification is sought. This process consists of 3 phases, documentation, evaluation, and rating. If the outcome of the rating phase is appropriate, certification is issued. If, on the other hand, the outcome is not appropriate, the certifying entity will list the errors detected. There are different organizations and entities for different activities. Certifying entities in Spain include Bureau Veritas and AENOR.
Implementation of a Quality Management System and ISO 9001:2015 Certification Process in a Dermatology DepartmentIn 2014, the dermatology department of the HGUA decided to obtain ISO 9001:2015 certification with the objective of improving its services.
In the article, we will explain the steps necessary to obtain certification and share some of our reflections on the experience.
To implement a QMS according to the requirements of UNE-EN-ISO 9001:2015 (the most recent version of the ISO standard)11 several stages are required:
1. Training. First, it is necessary to train the professionals in the principles and requirements of the ISO 9001:2015 standard through training sessions in the ISO process with the aim of familiarizing all personnel with the concepts and principles of quality, terminology, and requirements necessary for the process. On this point, it is often necessary to seek support from quality experts. Table 1 shows the main definitions and terminologies used in this system.
Main Definitions Used in the ISO-2015 Quality Management System.
| Audits | A test to check whether the actions performed for quality and its outcomes are consistent with the initial expectations or pre-established considerations |
| Client | Interested party who receives the product or service of the organization. It defines a person or entity that acquires the product or service from another |
| Continuous improvement | Instrument to contribute to the capacity to achieve the objectives of the organization |
| Corrective action | Actions to eliminate lack of compliance |
| Documented information | Information required to be controlled and maintained by an organization (3.01) and medium in which it is stored |
| FMAE | Failure mode and effects analysis. This is the procedure for analysis of potential failure in the classification system determined by the seriousness or effect of the system failures. |
| Indicator | Tool for monitoring and observing a system, constructed from the evaluation and resulting variables. There are structural, process, and outcome indicators |
| Key process | The key processes are those that add value for clients or directly affect their satisfaction or lack thereof. They form the value chain of the organization |
| Management | Person or persons accountable for governance at the highest level in an organization |
| Noncompliance | Failure to comply with a requirement |
| Opportunities | Opportune circumstance, moment, or means to perform or achieve something |
| Process | Set of activities that are mutually related or that interact with one another, and that transform inputs into outputs |
| Product | Outcome of a given process in the organization. These can be physical products or merchandise, or services. |
| Quality | Extent to which a set of inherent characteristics complies with the requirements |
| Quality Management System | Organizational structure, procedures, processes, and necessary resources to implement quality management |
| Quality manual | Set of documents that describe the quality system implemented by the service |
| Quality policy | Document that expresses the general objectives of the service with respect to quality, expressed formally by the highest level of management |
| Register | Record of the facts or any other information that is pertinent to the audit criteria and that can be verified |
| Risk | Effect of uncertainty |
| Scope | Limits and applicability of the management system |
| Strategic process | Process that enables the strategies and objectives of the organization to be defined and implemented. Strategic processes and common processes for most types of business |
| Support process | Methodology to follow for maintenance and to ensure the reliability of the equipment used to provide the service |
| SWOT | Tool for analysis of a situation in a company, institution, project, or person, taking into account Strengths, Weaknesses, Opportunities, and Threats. |
| Work environment | Set of working conditions in which the activities object of the organization are performed |
2. Quality committee. An internal quality committee should be set up within the dermatology department, with different professionals such as qualified dermatologists and residents, nursing staff from both the clinics and operating rooms, and administrative personnel. As with any committee, a president or secretary should be named. The quality committee should meet regularly. In the first working sessions, an in-depth analysis should be undertaken of the activity of the department, identifying the basic processes performed. Subsequently, a register should be made available for all members of the department to record incidents. These will be analyzed by the committee to identify lack of compliance and establish corrective actions and improvements.
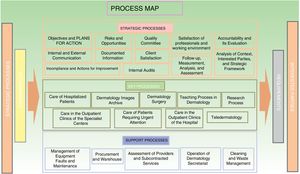
3. Initial diagnosis. The initial diagnosis is made though a SWOT (strengths, weaknesses, opportunities, and threats) analysis, detailing the different areas and activities of the department. This approach provides an overview of the current situation, and can be used to decide the scope of the activity to be certified. In our center, it was decided that the scope of the activity would be based on the diagnosis, treatment, teaching, and research in dermatology within the HGUA. Then, the main processes that occur in the department were to be identified. These include those denoted operational or strategic, and are common to any type of organization and enable its objectives to be defined. The key processes, that is, those corresponding to a given organization, are also identified. Finally, the support processes necessary for ensuring the sustainability and reliability of the equipment used for providing a service are identified. In our department, we identified 12 operational or strategic processes, 9 key processes, and 5 support processes (Table 2).
Types of Process: Strategic, Key, and Support for the Quality Management System.
| Strategic Processes |
| Analysis of context, interested parties, and strategic framework |
| Management of documented information |
| Management of risks and opportunities |
| Objectives and plans for action |
| Working of the quality committee |
| Management of accountability and its evaluation |
| Management of external and internal communication |
| Analysis of client satisfaction |
| Analysis of satisfaction of professionals and working environment |
| Follow-up, measurement, analysis, and evaluation |
| Management of internal audits |
| Management of lack of compliance and actions for improvement |
| Key Procedures |
| Care of patients requiring urgent attention |
| Care in the outpatient clinics of the specialist centers |
| Operation of dermatology operating room |
| Operation of the teledermatology process |
| Operation of the dermatology images archive |
| Operation of the teaching process |
| Management of the research process |
| Care in hospital clinics |
| Care of hospitalized patients |
| Support Processes |
| Management of equipment, faults and maintenance |
| Management of procurement and warehouse |
| Assessment of providers and subcontracted services |
| Cleaning and waste |
| Secretariat |
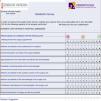
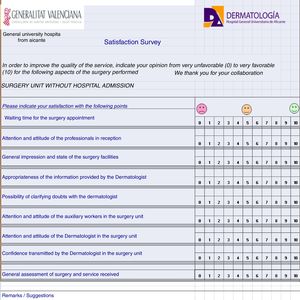
Strategic processes should be accompanied by corresponding registers. For example, in the process of operation of the quality committee, the functioning should be explained precisely and registers attached; these are used to record noncompliance, complaints, etc. For the processes related to client satisfaction and interested parties, it is necessary to record satisfaction and work environment surveys (see Fig. 2 for an example). The key processes should be analyzed with a failure mode and effects analysis (FMEA). This means that, for each process, it is necessary to know all the possible associated risks and the relevance of each one. Alongside the basic processes, the protocols for functioning of different activities can also be noted.
4. Task timelines. It is necessary to consider the different phases to be followed so that each person accountable for a certain process can elaborate the corresponding documentation. This will then be reviewed by members of the quality committee, with modification and adaptation as appropriate according to the consensus of all attendees. This process is also a means for disseminating information to other members of the department. The process is quite a painstaking one; thus, in our department, although the initial prevision was to perform the certification process in 2 years, this timeline was later extended by another year.
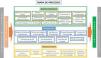
5. Quality system documentation. All the processes identified are documented in writing, following the design established by the standard, always mentioning the scope, process definitions, implementation of the process, and the associated indicators and registers. A date and version number are included, as well as a list of changes with respect to previous versions. All process documents are ordered and archived both as physical copies and electronically in a private space, housed in the web page of the department to which all accredited members have access with a password. The strategic processes, as well as the key and support ones, are integrated in a process map (Fig. 3), which represents a snapshot of the workings of the department. All this work is reflected in the quality policy and quality manual.
The quality policy is a definition that briefly covers the basis for the quality principles of the organization. In our case, it is expressed in the form of a small poster, visible everywhere within the dermatology department (Fig. 4).
The quality manual is the basic document of the QMS. It includes the vision and mission of the dermatology department, the quality policy, and the planning of objectives to implement that policy. The quality manual should be elaborated with reference to standards and comprises 10 chapters, which reflect the principles of ISO QMS based on the plan, do, check, act cycle. The first 3 chapters correspond to the introduction and should include the objectives, scope, and presentation of the organization, the pertinent standards, and the terms and definitions of the QMS. Chapters 4, 5, and 6 then cover the planning of the organization, with mention of context, leadership, and planning, respectively. Chapters 7 and 8, referring to support and the organization, are related to doing. Checking is addressed in chapter 9, which covers the execution. The manual closes with the actions for improvement. Table 3 shows the structure of the quality manual with the sections developed in each chapter, as well as the all processes, indicators, protocols, or registers included therein.
Structure of the Quality Manual.
| Chapters | Content | Associated Processes | |
|---|---|---|---|
| General Points | 1. Object and scope | Presentation of the organization | |
| 2. References to standards | |||
| 3. Terms and definitions | |||
| Planning | 4. Context of the organization | Scope SWOT analysis Quality management processes | KP StP SuP |
| 5. Leadership | Quality policy Roles Accountability | ||
| 6. Planning | Risk management FMAE Plans for action Changes | StP | |
| Do | 7. Support | Resources Accountability Knowledge Communication Documented information | StP |
| 8. Operation | Planning and control Requirements Development of products and services External services Internal services | KP PT | |
| Check | 9. Assessment of execution | Follow-up Measurement Analysis | R I |
| Action | 10. Improvement | Noncompliance | StP |
Abbreviations: I, indicators; KP, key processes; PT, protocols; R, registers; StP, strategic processes; SuP, support processes;.
6. Piloting the indicators and implementation of the requirements. The number of indicators that can be identified can be related to processes, outcomes, and structure. Structural indicators are those that confirm the existence of a certain procedure or protocol, which should be well structured, documented, and readily available. Process indicators show compliance with a certain aspect of the protocol and the outcome indicators are numerical and are intended to measure and compare. They serve to measure changes over time.
There may be numerous indicators and they may be dynamic. In our process, we identified 32 outcome indicators, 12 process indicators, and 19 structural indicators. Table 4 shows some of these indicators.
Examples of different types of indicators.
| Structural Indicators | Outcome Indicators | Process Indicators |
|---|---|---|
| Melanoma care | Total number of visits to dermatology department per year | Patients with surgical safety check-list |
| Accessibility to reference specialists (dermatologist, pathologist, oncologist) for patients with melanoma | Total number of first visits to dermatology department per year | Patients with informed consent in the medical records |
| Existence of a protocol for palmar hyperhidrosis | Total number of successive visits to hospital dermatology department per year | Patients with suspected melanoma lesions who undergo lesion excision in less than 30 days |
| Existence of a protocol for matricectomy with phenol | Total number of visits to dermatology department in the specialist centers per year | Patients with melanoma in advanced state with joint follow-up in dermatology and oncology |
| Existence of a protocol for severe psoriasis | Total number of first visits to dermatology department in the specialist centers per year | Patients with onychocryptosis who have undergone chemical matricectomy with phenol |
| Existence of a protocol for management of patients with hidrosadenitis | Total number of successive visits to specialist dermatology centers per year | Patients with individual register who have undergone therapeutic phototherapy protocol |
| Existence of a protocol for assessment of childhood hemangiomas by teledermatology | Ratio of number of successive and first visits to dermatology department in the specialist centers per year | Patients with psoriasis who undergo an assessment of severity with established indices |
| Existence of a protocol for management of atopic dermatitis | Mean time on waiting list for dermatology appointment | Patients with moderate or severe psoriasis who undergo measurement of quality of life |
| Existence of a protocol for hand eczema | Mean delay in dermatology appointments | Patients with photodynamic therapy with complete record of response in medical records |
| Existence of a protocol for spontaneous urticaria | Number of biopsies | Percentage of patients referred through teledermatology with resolution of consultation |
| Existence of a protocol for photodynamic therapy | Number of phototherapy treatments | Percentage of patients with serious diseases referred through teledermatology who underwent surgery |
| Existence of a protocol for phototherapy | Total number of surgical interventions performed by the dermatology department per year | Percentage of patients referred through teledermatology with follow-up in the dermatology clinics |
7. Conduct of an internal audit. Once the information has been collected, all processes should undergo an internal audit, which consists of a simulation of the definitive audit process with the aim of detecting possible failures. In our case, this process detected several points of lack of compliance that were corrected before the external audit, in October 2017.
8. Conduct of the external certification audit. The external audit is a 2-phase process. In the preliminary phase, the documentation is checked. In the final phase, on 2 consecutive days, the auditors review and compare all documentation produced and perform a physical check of all areas of the clinics, operating rooms, specialty centers, with interviews with different members of the department, to verify the authenticity of the processes documented.
Once this audit is complete, a report is issued. In our case, we received a positive opinion on January 30, 2018, and ISO 9001:2015 certification was awarded for the care, research, and teaching of the dermatology department of the HGUA (Fig. 5).
9. Annual review and renewal of the certificate every 3 years. After obtaining the initial certificate, all procedures should be reviewed annually and the certificate should be renewed every 3 years.
Finally, the Conselleria de Sanitat de la Comunitat Valenciana (Valencian Health Authority) has a registry of quality certifications. In our case, after the appropriate paperwork, the quality certificate of the dermatology department of the HGUA was included in this registry in May 2018.
DiscussionAfter the experience of implementation of QMS in the dermatology department and obtaining the ISO 9001:2015 certification, we would like to comment on the following aspects:
What Does It Mean to Have an ISO Certificate?The ISO quality certification means that the organization with the seal of quality is committed to a QMS based on accountability, good management, and continuous improvement, 3 essential requirements to offer a good service.
The benefits of implementing an ISO 9001:2015 QMS can be both internal and external.
Internally, having a QMS indicates that the necessary tools and procedures have been established to guarantee the quality of the services offered. It means that all processes have been systematized and standardized and that a collective effort has been made to provide up-to-date documentation, collecting material from different sources, optimizing resources, and detecting failures. It implies great commitment from all professionals to achieve a satisfactory level of work and ensures cohesion of all levels of the organization in working for a common objective. The decision to undergo control and evaluation of a QMS such as the ISO standard is a voluntary process that requires the organization to be bold enough to submit to external quality assessments, and then to commit to correcting or restructuring irregularities or anomalies detected during the audits if the standards are not met.
Externally, for the users, the process may increase the confidence in the department and satisfaction of the patients and other interested parties, both in the hospital and in other parts of health care, knowing that the services on offer comply with established quality requirements, accredited through external control.13
For the health center that obtains certification, there are some differences in what this may mean, depending on whether the center is in the public or private sector. In private health centers, ISO certification is normal practice, as it represents a seal of quality for the care provided and adds to the prestige of the clinic. It is also an important marketing tool, useful for positioning in the market and a factor that might differentiate a center from others without certification. However, in a clinic in the public sector such as ours, the greatest benefit is that it shows that quality standards are met in the care provided and the research and teaching performed. These standards had not been documented previously and the process demonstrates a commitment to provide high quality health care. The process of certification in public health clinics is more usual in centralized services such as radiology, nuclear medicine,14,15 and clinical laboratories, where it is almost an essential requirement to accredit compliance with quality standards. In clinical services, such compliance is not essential to demonstrate, and so it is not usually the case that an isolated department opts for this type of certification,13,16 although some health centers do seek overall certification, which covers all their departments. To the best of our knowledge, no other dermatology department in the public sector has sought individual certification.
The main drawback of developing a QMS is the great effort that is required for implementation. It is necessary to identify all the activities related to quality, determine accountabilities, and divide up the work. The situation of the department needs to be analyzed, with its projection, strengths, and weaknesses to propose short- and medium-term objectives. A high degree of self-criticism with the activity being performed is necessary in order to detect when the pre-established standards are not met and to take remedial action. It is a laborious process which requires recording, testing, and honing for several months, as well as an internal audit, before the hospital requests the certification audit.
Another drawback is the excessive paperwork, as it is necessary to document all the processes and put in writing all the procedures linked to management, standards, and the guidelines for operation to facilitate compliance and transmission.
Finally, another added difficulty is that it is necessary to become familiar with the language used in quality management and specifically the ISO QMS. This language will sound strange initially. In addition, technical advice may be needed.13 Finally, the costs of acquiring certification are another drawback for a department in the public sector, given that the difficulties for obtaining funding are a major obstacle.
ConclusionIn our experience, implementation of the QMS has not essentially changed the working methods of our department but it has enabled a reorganization of the system. The main processes have been identified and put in writing, assisting in compliance and supervision of the stages and the personnel involved.
The system improves the safety of the patients through an increased control of the activity performed,13 allows a greater satisfaction for all interested parties, internal recognition at the institutional level of the hospital or other fields of health care, increased visibility to other sectors of the society, and, above all, recognition from the patients, who feel encouraged to place their confidence in us when they are attended in the department that they know has been subject to an exhaustive quality assessment.
ISO certification of a dermatology department that provides patient care and performs research and teaching activities in the public health sector is also cause for satisfaction, bearing in mind the high demands for complying with ISO quality standards.
In short, a department that embarks on a quality certification process such as ISO 9001:2015 has to be firmly convinced of the need to provide quality service in the public health sector.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
The authors would like to thank all members of the dermatology department of the Hospital General of Alicante, Spain, for their support and commitment to the project and Joaquín Uris and Marina Albert, of Uris-21 Consultores, for technical support.
Please cite this article as: Betlloch-Mas I, Ramón-Sapena R, Abellán-García C, Pascual-Ramírez JC. Implantación y desarrollo de un sistema integrado de gestión de calidad según la norma ISO 9001:2015 en un Servicio de Dermatología. Actas Dermosifiliogr. 2019;110:92–101.