The diagnosis of disorders of the hair and scalp can generally be made on clinical grounds, but clinical signs are not always diagnostic and in some cases more invasive techniques, such as a biopsy, may be necessary. This 2-part article is a detailed review of the histologic features of the main types of alopecia based on the traditional classification of these disorders into 2 major groups: scarring and nonscarring alopecias. Scarring alopecias are disorders in which the hair follicle is replaced by fibrous scar tissue, a process that leads to permanent hair loss. In nonscarring alopecias, the follicles are preserved and hair growth can resume when the cause of the problem is eliminated. In the second part of this review, we describe the histologic features of the main forms of scarring alopecia. Since a close clinical-pathological correlation is essential for making a correct histopathologic diagnosis of alopecia, we also include a brief description of the clinical features of the principal forms of this disorder.
El diagnóstico de las enfermedades del cabello y del cuero cabelludo se basa, en la mayoría de las ocasiones, en el reconocimiento de signos clínicos; sin embargo, dichos signos no siempre son característicos y, en ocasiones, tenemos que recurrir a técnicas más invasivas como la realización de una biopsia. En este artículo se revisan de forma detallada las principales formas de alopecia desde un punto de vista histopatológico, y para ello se utiliza la clasificación tradicional de las alopecias que las divide en 2 grandes grupos: las alopecias cicatriciales y las no cicatriciales. Las alopecias cicatriciales son aquellas en las cuales el folículo piloso es sustituido por tejido fibroso cicatricial, causando una pérdida permanente del cabello. En las alopecias no cicatriciales el folículo permanece intacto y puede retomar su actividad cuando cesa el estímulo desencadenante. La segunda parte de este artículo revisa las principales formas de alopecia cicatricial desde un punto de vista histopatológico. Dado que una buena correlación clinicopatológica es fundamental para realizar el correcto diagnóstico histopatológico de las alopecias, en este artículo se incluye también una breve descripción de las características clínicas de las principales formas de alopecia.
In our daily clinical practice, dermatologists often encounter patients consulting for alopecia. In many cases, correct diagnosis of these conditions can be made from the presentation and course of hair loss. However, sometimes, a biopsy is necessary to enable a definitive diagnosis to be established. This article reviews in detail the main forms of cicatricial alopecias from a histopathological standpoint.
Cicatricial AlopeciasCicatricial alopecias are a group of conditions in which the hair follicles are replaced by vertical fibrotic tracts or hyalinized collagen, giving rise to permanent hair loss. This process manifests clinically as the loss of follicular ostia and cutaneous atrophy. There are many causes of secondary cicatricial alopecia, such as infiltrative processes (cutaneous metastasis, sarcoidosis), trauma (burns, radiation), and infections. However, the term cicatricial alopecia is used mainly to refer to primary cicatricial alopecias (PCAs), a group of diseases in which the hair follicle is the main target of the inflammatory process while the interfollicular dermis is spared.1 Classification of cicatricial alopecias is confusing and controversial given that the etiology, in many cases, is unknown and the clinico-pathological characteristics overlap, vary over time, and depend on racial and genetic factors. In this article, we will analyze the classification established in 2001 by the North American Hair Research Society (NAHRS), which classifies PCAs according to the composition of the inflammatory infiltrate into lymphocytic, neutrophilic, and mixed types (Table 1).2
Classification of Primary Cicatricial Alopecias (PSA) According to the North American Hair Research Society (NAHRS).
| Main Composition of the Inflammatory Infiltrate | Entities |
|---|---|
| Lymphocytic | Chronic cutaneous lupus erythematosus (CCLE) |
| Lichen planopilaris (LPP) | |
| Pseudopelade of Brocq | |
| Central centrifugal cicatricial alopecia (CCCA) | |
| Alopecia mucinosa | |
| Keratosis follicularis spinulosa decalvans (KFSD) | |
| Neutrophilic | Folliculitis decalvans (FD) |
| Dissecting cellulitis (DC) | |
| Mixed | Acne keloidalis nuchae (AKN) |
| Acne necrotica varioliformis | |
| Erosive pustular dermatosis | |
| Nonspecific |
PCAs have an initial active phase, with more or less specific clinical characteristics. Scarred areas progressively start to appear, usually in the central area of the lesions. In advanced phases of the disease, differential diagnosis using clinical manifestations is therefore much more difficult if not impossible at times. Biopsy samples will be useful for establishing or confirming the diagnosis. For the sample to be representative, it should be taken from areas in which follicular ostia can still be observed. It is particularly useful to take a biopsy from the periphery of the lesions, areas of positive hair pull test, and areas of visible clinical activity. In addition, the diagnostic yield can be improved by taking the biopsy parallel to the follicle in order not to section it. If a single biopsy is taken, it is best to request transversal sectioning to enable multiple follicles to be observed at different levels.3,4 However, such an approach does not allow observation of the epidermis, dermis, and pilosebaceous units as a whole, something which can be of great use in this type of alopecia.3 Therefore, faced with the suspicion of cicatricial alopecia, the best approach is to take 2 samples, one for transverse sectioning and the other for vertical sectioning. Moreover, staining for elastic fibers and direct immunofluorescence (DIF) can also be useful in the histopathological study of scarring alopecias. The key histopathological findings of use in the diagnosis of cicatricial alopecia are summarized in Table 2.
Key Histopathological Features of Primary Cicatricial Alopecias.
| Type of Alopecia | Key Histopathological Features |
|---|---|
| Chronic cutaneous lupus erythematosus (CCLE) | Vacuolar-type interface dermatitis |
| Folliculocentric lymphocytic inflammatory infiltrate | |
| Dilated infundibula containing laminar keratin | |
| Destruction of perifollicular elastic fibers | |
| IgG and C3 deposits at the interface between the dermis and the follicular epithelium | |
| Lichen planopilaris (LPP) | Lichenoid-type interface dermatitis |
| Folliculocentric lymphocytic inflammatory infiltrate | |
| Concentric lamellar fibrosis | |
| Cradle cap scar with destruction of elastic fibers | |
| Pseudopelade of Brocq (PB) | Diagnosis by exclusion in absence of findings characteristic of other cicatricial alopecias |
| Central centrifugal cicatricial alopecia (CCCA) | Lamellar fibroplasia around follicular infundibula |
| Fibrous tracts replacing pilosebaceous unit | |
| Thickened elastic fibers in the dermis | |
| Alopecia mucinosa | Mucin deposits in the outer root sheaf |
| Lakes of intrafollicular mucin | |
| Keratosis follicularis spinulosa decalvans (KFSD) | Compact hyperkeratosis in the infundibulum |
| Mixed neutrophilic/lymphocytic inflammatory infiltrate | |
| Primary Neutrophilic Cicatricial Alopecias | Intrafollicular and perifollicular neutrophilic inflammatory infiltrate |
| Perifollicular and interstitial fibrosis | |
| Several hair shafts emerging from a follicular infundibulum (tufted hair folliculitis) | |
| Foreign-body type granulomatous reaction to broken hair shafts. |
Patients with a variant of lupus erythematosus may present involvement of the scalp. Those with chronic cutaneous lupus erythematosus (CCLE), especially those with chronic discoid lupus erythematosus are, however, the only ones who progress to a cicatricial alopecia. In agreement with Sperling,5 we prefer the term CCLE to discoid lupus erythematosus and this will be the term that we use in our descriptions. Patients with systemic lupus erythematosus can present with CCLE lesions, although most patients with CCLE do not have systemic disease. Among the patients with cutaneous involvement only, approximately 50% will have scalp involvement and very few will develop systemic disease. Clinically, these patients present erythematous and desquamative plaques of alopecia on the scalp, with epidermal atrophy. Dilated follicular infundibula are present with horny plugs. These plaques show a centrifugal growth, and several plaques can coalesce to form areas of cicatricial alopecia with irregular borders. The presence of a central hypopigmented area with peripheral hyperpigmentation is characteristic of advanced CCLE lesions in patients with medium-high phototype.
On histopathology, active CCLE lesions on the scalp show a vacuolar-type interface dermatitis and a folliculocentric inflammatory lymphocytic infiltrate. This dermatitis tends to involve the interfollicular epidermis, although this structure may sometimes be spared, thereby complicating the diagnosis.4 A common finding in CCLE, although also observed in lichen planopilaris (LPP), is the presence of colloid bodies, hyaline bodies, or Civatte bodies, as well as the presence of dyskeratosis in the follicular epithelium and the epidermis. It is common to observe dilated infundibula with laminar keratin present inside. Such a finding is common in PCAs in general, and clinically, this is manifest as the presence of horny plugs under physical examination. The folliculotropic inflammatory infiltrate is characteristically distributed around the infundibulum and the isthmus,6 although it can also involve the entire follicle. This inflammatory infiltrate, which is predominantly lymphocytic although it often includes plasma cells, also has a superficial and deep perivascular pattern with a striking perieccrine distribution.4,6–9 Moreover, atrophy and destruction of the sebaceous glands are often observed from early stages.7,9 In the late stages, CCLE lesions show striking lamellar fibrosis, surrounding the upper follicle, although these lesions can also be panfollicular, and as the condition progresses, the follicle may be completely destroyed (Fig. 1). As in other PCAs, foreign-body granulomas can be observed around free hair shafts lacking epithelial lining in the dermis.7 In addition, interstitial mucin is often observed in the reticular dermis, and on occasions, the formation of germinal center lymphoid follicles is noted in the hypodermis.10
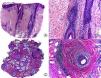
Cicatricial alopecia in a plaque of chronic cutaneous lupus erythematosus. A, Few follicular units are observed in longitudinal sections (hematoxylin and eosin [HE] x10). B, Fibrosis around follicular remnants (HE x200). C, Same case with transversal sections (HE x20) D, Concentric fibrosis around follicular remnants (HE x200).
Staining for elastic fibers can help in the differential diagnosis of CCLE with other PCAs. Advanced CCLE lesions show destruction of perifollicular elastic fibers,11 whereas in the late LPP lesions, there is a cradle cap scar in the superficial dermis, with destruction of elastic fibers only in this area.11 In the pseudopelade of Brocq (PB), elastic fibers are not only destroyed but also appear notably thickened.11
DIF is also useful in the differential diagnosis of CCLE with other PCAs. A biopsy sample should ideally be taken from a lesion with onset at least 2 to 3 months earlier that has not been treated for at least 3 weeks.12 On performing DIF in lesions with CCLE, IgG and C3 or IgM deposits are observed in a granular pattern or in a homogeneous band at the dermal-epidermal junction and the interface between the dermis and follicular the epithelium.5,8,13 IgA is present less frequently.5,13 In one study, a higher percentage of CCLE lesions in the scalp were positive in DIF when compared with biopsies taken from other anatomical regions.12 Therefore, it is recommended to take a fresh biopsy for DIF whenever it is suspected that CCLE is among the conditions to be considered in a differential diagnosis for PCA.
Finally, differential diagnosis of LPP and CCLE can be a challenge in some cases, especially when CCLE does not affect the surrounding epidermis. In these cases, the presence of inflammatory infiltrate with plasma cells and deep and perieccrine perivascular involvement, as well as the presence of dermal mucin and colloid bodies at the dermal-epidermal junction provide further support for diagnosis of CCLE.14 Moreover, the presence of a periodic acid schiff-positive thickened basement membrane is a classic finding and often seen in chronic CCLE lesions,6,13 and may even be present in very advanced lesions.6 Such observations can be of great help in the differential diagnosis of these 2 processes.3
Lichen PlanopilarisLPP is the name used to define lichen planus when there is involvement of the hair follicles. It is divided into 3 types according to the clinical presentation: classic LPP, frontal fibrosing alopecia (FFA), and Graham Little syndrome. From the histopathological point of view, these 3 processes are almost indistinguishable, even in their active phases, and so they are described together. The clinical presentation of classic LPP on the scalp is very similar to that of CCLE. Large plaques with keratotic follicular papules and spiny follicular hyperkeratosis are present, in contrast to CCLE, in which the greatest activity is observed in the peripheral area,8,15 characteristically sparing follicles within the plaque of alopecia. The classic polygonal lesions in lichen planus are not observed in the scalp but they may be present in other body areas, and in that case, they may be of assistance in the diagnosis. Likewise, the typical mucosal or nail lesions in lichen planus may also support the diagnosis. The coexistence of LPP and vulvar lichen planus has been reported, for example.16 FFA is considered a variant of LPP with a specific pattern, which gives rise to regression of the frontotemporal hairline and loss of eyebrows as the clinical manifestation.17–21 Finally, Graham Little syndrome is the term used for the triad of cicatricial alopecia of the scalp, keratotic follicular papules on the trunk and limbs, and reversible loss of pubic and/or axillary hair.
Histopathologically, a lymphocytic interface dermatitis, usually of the lichenoid type, is observed in active lesions of LPP. This lesion spares the epidermis and interfollicular dermis and usually has perifollicular involvement (Fig. 2). As in the clinical manifestations, not all hair follicles are found to be affected in the biopsy.6,7,21 The lichenoid infiltrate predominantly affects the permanent part of the follicle (infundibulum and isthmus), and may obscure the interface between the adventitial dermis and follicular epithelium,6,7,13,22–24 giving rise to an image in which the infiltrate and periinfundibular fibroplasia are strangling the infundibulum.8 Above the infiltrate, the infundibulum appears dilated, and takes on a funnel-like appearace,8 with hypergranulosis and layers of keratin, basophils, and orthokeratosis inside.22,33 These correlate with the clinical findings of spiny hyperkeratosis. The presence of several colloid bodies, made up of dyskeratotic keratinocytes, which are positive for cytokeratin stains, is noteworthy along the entire dermo-epidermal junction.22–24 As is the case with CCLE, sebaceous glands are atrophic or completely destroyed from the initial phases of the process.7,8,23 The deep vascular plexus, as well as other adnexal structures are not affected, and mucin deposits in the dermis are not a typical finding in LPP. At times, typical findings of lichen planus are observed in the biopsy,22,23 and these can be of great help in assisting the histopathologic diagnosis.
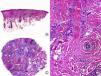
Lichen planopilaris. A, Perifollicular infiltrate is observed in longitudinal sections (hematoxylin and eosin [HE] x10). B, At higher magnification, lymphocytes are observed scattered in the follicular epithelium (HE X200). C, Same case studied with transversal sections (HE x20). D, At higher magnification, perifollicular concentric fibrosis and peripheral lymphocytic infiltrate are observed (HE x200).
As the condition progresses, concentric lamellar fibrosis is observed,23 along with destruction of the hair follicles to be replaced by thick longitudinal fibrous tracts and the appearance of foreign-body granulomas.24 Fibrosis has a limited presence in the adjacent tissue, but is more prominent in the papillary dermis and associated with epidermal atrophy.23 Staining for elastic fibers will show a cradle cap scar centered on the follicle. The histopathologic changes in FFA and Graham Little syndrome cannot be differentiated from classic LPP,17,18 although the follicular triad has been reported. This consists of simultaneous involvement of different types of follicles (terminal, intermediate, and vellus) at different stages of the follicular cycle (anagen, catagen, and telogen) as a key histopathologic finding in the diagnosis of the initial phases of FFA.25
The most difficult histopathologic differential diagnosis of LPP is with CCLE. The clinical-pathological correlation is particularly important in this respect. In particularly difficult cases, DIF may be of some use. In LPP, the abundant Civatte bodies are positive for IgM and less frequently for IgA, IgG, and C3, and they predominate in the follicular epithelium of the infundibulum and the isthmus.22,26,27 This finding, although a characteristic highly suggestive of LPP, is not pathognomonic, as it can also be observed in CCLE.27 However, it seems that the two processes can be differentiated according to the composition of these Civatte bodies, which are formed of necrotic keratinocytes (expressing cytokeratins) in LPP and by aggregates of the basement membrane (positive for collagen IV) in CCLE.
Pseudopelade of BrocqThere is much debate as to whether PB is a nosological entity or just the noninflammatory end stage of other PCAs. The term has been widely used in the dermatology literature ever since the first description by Brocq in 1885.28 Several studies have attempted to clarify whether PB is a separate entity or not. Some authors clinically diagnose this entity in all patients who do not meet the criteria for LPP or CCLE, and they report that between 33%29 and 69% (this latter percentage for early and active lesions)30 may be diagnosed histopathologically as CCLE or LPP. In the most recent classification of the NAHRS in 2001, PB was described as a specific entity.2 Sellheyer and Bergfeld31 also considered PB as a separate entity, pointing out that there is a clear clinical and histopathologic absence of keratin plugs, and that the lesion retains the dermal network of elastic fibers. None of these findings are observed in LPP or CCLE.
Clinically, the condition presents as small flesh-colored patches of alopecia without hyperkeratosis or signs of inflammation.29 The plaques show a certain degree of atrophy, giving rise to the classic description of footprints in the snow.32 The vertex and parietal regions are more frequently affected.29
From an histopathologic point of view, PB is characterized by the absence of interface dermatitis, unlike LPP or CCLE, but distinctive histopathologic features of PB have not been reported.33 Early lesions present scant or moderate perifollicular lymphocytic infiltrate, which predominates in the periinfundibular region.29 Sebaceous glands are destroyed early in the process.34 As the condition progresses, lamellar fibroplasia appears around the follicular infundibula, leading to complete destruction of the pilosebaceous unit, with the appearance of fibrous tracts in its place (Fig. 3). The arrector pili muscle remains intact and foreign body granulomas can be observed around the hair shafts.29 In PB, staining for elastic fibers shows these structures to be notably thickened both in the adventitial and reticular dermis,11 an observation that assists in the differential diagnosis with other PCAs in advanced phases.
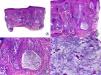
Longstanding pseudopelade of Brocq. A, Low-magnification view showing columns of fibrosis replacing the follicles (hematoxylin and eosin [HE] x10). B, Detail of the previous image showing vertical fibrosis mixed with actinic elastosis (HE x200). C, Transversal section of the same case, showing several follicular units (HE, X20). D, At higher magnification, concentric fibrosis around follicular remnants can be observed (HE x200).
Central centrifugal cicatricial alopecia (CCCA) is a term coined by the NAHRS consensus group. It is defined as hair loss starting at the vertex region, and extending in a centrifugal pattern.2 It is a descriptive term that is used to group entities such as the follicular degeneration syndrome, pseudopelade in African-Americans, and central elliptical pseudopelade in Caucasians.2 There is substantial overlap in histopathological terms with PB, but the clinical presentation is different so clinico-pathologic correlation is essential for diagnosis of the 2 entities.
Its pathogenesis is unknown. Sperling et al35,36 postulated that the process is the result of early degeneration of the inner root sheath leading to damage to the outer root sheaf by the hair shaft, triggering a chain of histopathological events that culminate in the scarring process. These authors considered that the finding of this premature degeneration of the inner root sheath, in absence of signs of inflammation, was very suggestive of follicular degeneration syndrome.36 However, Gibbons and Ackerman,37 Headington,7 and Sulllivan and Kossard10 do not consider CCCA to be an independent nosological entity and suggest that the histopathological changes present are nonspecific and similar to other PCAs. In fact, Ackerman et al.38 considered that CCCA is really the end stage of a traction alopecia.
The histopathological characteristics of CCCA have not been extensively described in the literature.4,8,35 In general, the findings reported are similar to the those of PB. A perifollicular lymphocytic infiltrate is observed around the upper part of the follicle, and sometimes in the perivascular region. Some authors have reported an asymmetric narrowing of the follicular wall, which is best observed in transversal sections, and this significantly displaces the hair shaft to an excentric location.39 As the lesions progress, lamellar fibroplasia is observed, as well as destruction of the pilosebaceous units, giving rise to the development of cicatricial tissue in place of preexisting follicles. Staining for elastic fibers shows a similar pattern to PB.11
Alopecia MucinosaAlopecia mucinosa is an inflammatory process of the pilosebaceous unit that can be related to both permanent and reversible alopecia. The name refers to the main histopathological finding, the presence of intrafollicular mucin. This finding is considered a nonspecific reactive histopathological pattern,40,41 and the denomination of follicular mucinosis appears to be appropriate.40–42 Traditionally, alopecia mucinosa is classified in 2 types, a primary idiopathic form and another secondary to lymphomas. Given the large degree of overlap between the 2 entities, and given that cases of primary alopecia mucinosa that progress to lymphoma have been reported,42–44 the distinction might be artificial. Indeed, primary and secondary alopecia mucinosa could represent different aspects of a single disease spectrum. Primary alopecia mucinosa would thus be considered as a premalignant condition,45 or as an indolent form of mycosis fungoides (MF) with good prognosis.46 Clinically, both forms are characterized by presenting as grouped follicular papules, erythematous patches, and/or fluctuating plaques that more often affect the head and neck, but trunk and limb involvement has also been reported.46
From the histopathological point of view, the earliest abnormality observed is mucin deposition between keratinocytes of the outer root sheath (Fig. 4). Intense deposition may give rise to the formation of mucin lakes that affect the entire follicle as well as the sebaceous gland.8,46 A perifollicular and intrafollicular lymphocytic infiltrate is observed, with lymphocytes of both normal and atypical appearance. Lymphocyte exocytosis can be observed in the follicular epithelium in the primary form.46 Involvement of the dermis is variable, with a lymphocytic infiltrate with a superficial and profound perivascular pattern, as well as a diffuse pattern.8 As in the clinical presentation, there are no reliable and reproducible histopathological characteristics to differentiate between primary alopecia mucinosa and the secondary lymphoma-associated form. Moreover, cellular atypia and monoclonal rearrangement of T cell receptor genes can be found in both forms, and so such observations are not useful for differential diagnosis.46 In late stages, destruction of the pilosebaceous unit occurs, and residual tracts of mucin persist cuffed by inflammatory cells.47 Unlike most PCAs, concentric lamellar fibrosis is not observed in alopecia mucinosa.8 Routinely, staining for mucin is required. However, when we observe prominent spongiosis in the follicular epithelium, it is important to carry out differential diagnosis with atopic dermatitis; thus, the use of stains may be useful.31 Eosinophilic folliculitis can also present with follicular mucinosis, but other additional histopathological characteristics are present to aid in differential diagnosis.31
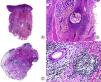
Alopecia mucinosa. A, Low-magnification view (hematoxylin and eosin [HE] x10). B, At higher magnification, lakes of mucin can be observed within the hair follicles (HE x20). C, Still higher magnification view showing mucin among the keratinocytes of the follicular epithelium and a large lake of mucin (HE x200). D, Detail of the granular basophilic material within the follicular epithelium (HE x 400).
Keratosis follicularis spinulosa decalvans (KFSD), also known as keratosis pilaris decalvans or ichthyosis follicularis, is one of 3 entities that are included under the term keratosis pilaris atrophicans.48,49 The other 2 entities predominantly affect the face; these are keratosis pilaris atrophicans faciei and atrophoderma vermiculata. KFSD is considered an inherited X-linked genodermatosis.50
Clinically, it presents with areas of alopecia that show hyperkeratotic follicular papules and pustules. Onset often occurs during adolescence,48,49 and involvement is predominantly of the scalp, although the eyebrows and eyelashes can also be affected. In the scalp, areas already affected by alopecia can have residual keratin plugs, surrounded by perifollicular erythema, as well as more striking punctate atrophy on the face.49 Keratosis pilaris on the trunk and limbs, corneal dystrophy, and photophobia may also be associated with plaques of alopecia.
KFSD is classified by the NAHRS as a lymphocytic alopecia. However, although this is true in advanced disease or end stages,48 the initial lesions also show a neutrophilic infiltrate.49 The initial defect seems to be abnormal keratinization that gives rise to hypergranulosis and compact hyperkeratosis in the upper part of the infundibulum,49 which correlate clinically with follicular plugs. In the next phase of acute inflammation, spongiosis appears along with a neutrophilic infiltrate in the infundibulum and adjacent epidermis. The course includes the appearance of a lymphocytic infiltrate associated with perifollicular fibrosis, predominantly in the upper part of the follicle. In the end stages, fibrosis is observed with the presence of foreign-body granulomas surrounding the hair shafts, as well as destruction of the hair follicle.31
Primary Neutrophilic Cicatricial AlopeciasThe category of primary neutrophilic cicatricial alopecias covers folliculitis in which the initial neutrophilic infiltrate is of importance in pathogenesis. In these cases, bacterial superinfection of the follicle and the consequent neutrophilic inflammatory response will be the basis for the clinical and histopathological findings. With the subsequent progression of the condition and the appearance of fibrosis, the infiltrate becomes a mixed.
In the past, substantial clinical differences have been reported among the processes included within this group of alopecias. However, the histopathological findings are very similar, thus questioning if these are really distinct processes or just different stage within the same spectrum of lesions. With this possibility in mind, we will now review each entity separately to maintain the traditional nomenclature.
Folliculitis DecalvansFolliculitis decalvans (FD) is a chronic and progressive pathological process characterized by destructive suppurative folliculitis. Clinically, it presents as plaques of alopecia with follicular pustules at the margins, where the lesion is active.8,13,51 The plaques predominate in the scalp, but they can also appear in other regions of the body with terminal follicles. On resolution, they leave a central scar.
Histopathologically, when a biopsy is taken from the active border, it is possible to observe an acneiform dilatation of the follicular infundibulum, associated with an intrafollicular and perifollicular neutrophilic inflamatory infiltrate.13,51 With progression, the infiltrate will affect the entire follicle and will be made up mainly of lymphocytes and histiocytes, with plasma cells and multinucleated giant cells present. In addition, perifollicular and interstitial fibrosis is observed, with dermal involvement that is not directly perifollicular. Sullivan and Kossard10 consider that the presence of plasma cells may be a key for the diagnosis of FD in its advanced states (Fig. 5). The final stages are characterized by the presence of fibrous tracts that replace the hair follicles. In addition, foreign-body granulomas can be observed around the hair shafts in direct contact with the dermis.
Folliculitis decalvans. A, Low magnification view showing several broken hair follicles with perifollicular fibrosis (hematoxylin and eosin [HE] x10). B, Detail of the previous image showing an infundibular cyst surrounded by inflammatory infiltrate and below a pigmented hair shaft surrounded by multinucleated giant cells (HE x 200). C, Transversal sections showing perifollicular fibrosis and cuffs of infiltrate around the hair follicles (HE x20). D, At higher magnification, the infiltrate can been seen to be made up of lymphocytes and plasma cells (HE x200).
At times, special staining may be necessary to rule out a microbial etiology. The main histopathological differential diagnosis is with dissecting cellulitis/folliculitis, which presents with absence of fistulous tracts and the interstitial infiltrate is only observed in advanced states. To help differentiate FD from acne keloidalis, the presence of fibrosis with hypertrophic scarring is only observed in the latter entity.
Tufted hair folliculitis (THF) is a suppurative folliculitis in which multiple hair shafts emerge from a single follicular infundibulum.51,52 Often, cultures are positive for Staphylococcus aureus and the condition can coexist with FD. Some authors think that FP is pathognomonic of FD,51 although the general consensus is that this is a nonspecific form of cicatricial alopecia,52,53 as it can be found in many primary and secondary cicatricial diseases. Histopathologically, a dilated infundibulum is observed with several hair shafts inside that emerge at the surface through a common follicular ostium.
Dissecting Cellulitis/FolliculitisDC, initially known as perifolliculitis capitis abscedens et suffodiens, is a suppurative folliculitis, and so cellulitis is not an appropriate terminology. It is an entity considered within the tetrad of follicular occlusion, along with acne conglobata, hidradenitis suppurativa, and pilonidal cysts. These entities are characterized by abnormal follicular keratinization that gives rise to obstruction of the hair follicle, with secondary bacterial infection and subsequent destruction of the hair follicle.54 Clinically, DC presents as deep inflammatory nodules that from the outset can cause alopecia in the overlying scalp. As the disease progresses, fluctuant plaques appear connected to one another by fistulous tracts, which can express purulent exudate through several ostia at the same time. The deep involvement can cause a cerebriform appearance of the scalp.
In the initial lesions, dilatation of the infundibula is observed, and these can appear obstructed by horny plugs. Numerous neutrophils are present within the infundibula, leading to perforation of the follicular epithelium, with the subsequent formation of dermal and subcutaneous abscesses, which appear connected to one another by fistulous tracts coated with stratified squamous epithelium. This epithelium is derived from the outer root sheath of the proliferating follicle, and constitutes the main histopathological finding in this entity.7,13,51 In the most advanced stages, the infiltrate is of a mixed type, with the presence of lymphocytes, plasma cells, and foreign-body type giant cells. In this stage, extensive fibrosis is also present, surrounding the abscesses and fistulous tracts, with destruction of the hair follicles and subsequent cicatricial alopecia.
The presence of fistulous tracts enables differential diagnosis of this entity to be established with other types of suppurative folliculitis of the scalp. However, the differential diagnosis with hidradenitis suppurativa is not possible based on histopathological findings. The different sites of the lesions in both entities can help us to make a definitive diagnosis.
Primary Mixed Cicatricial AlopeciasAcne Keloidalis NuchaeThe term acne keloidalis nuchae (AKN) is a misnomer as it refers to an entity that is not related to acne vulgaris and is not characterized by keloid lesions but rather hypertrophic lesions.55 Clinically, this condition is characterized by the presence of millimetric follicular papules, which are firm to touch, can be scabby, umbilicated, or pustular, with hair within them.51,56 The papules-pustules can give rise to plaques with a keloidal appearance and nodules that can present purulent secretion. This condition predominantly affects black individuals.
From an histopathologic point of view, inflammatory folliculitis is observed in which the inflammatory infiltrate is situated in the lower part of the isthmus. This infiltrate is granulomatous and is associated with a neutrophilic and lymphocytic infiltrate, occasionally with plasma cells in the upper and mid part.56,57 Sebaceous glands are destroyed in the early stages of this process, with an abundant inflammatory infiltrate in the surrounding area when incipient lesions are biopsied.56,57 It has been postulated that inflammatory involvement of the infundibulum, which damages its epithelium, triggers an attempt to repair the damage in the form of lamellar fibroplasia. However, this process is not usually effective, and the damaged follicle finally releases its hair shaft to the surrounding dermis leading to an acute and chronic granulomatous reaction, responsible for the clinical manifestation of papules with a firm consistency. The damaged hair shafts cannot be eliminated because of the involvement of the upper part of the follicle, thereby increasing the inflammatory and granulomatous reaction, with a continuous reparative process triggering that finally leads to hypertrophic scarring.56,57 Keloid-type streamers of collagen may infrequently be observed.55–57
Acne Necrotica VarioliformisAcne necrotica varioliformis is a rare dermatosis that presents as a necrotizing disorder of the hair follicle and gives rise to the appearance of varioliform scars. Clinically, it is characterized by repeated outbreaks of follicular papules-pustules with central necrosis in adult patients. These lesions leave a depressed scar.58 The lesions are observed mainly in the frontal hairline, but also in other seborrheic regions of the face.58,59
Histologically, the initial lesions present spongiosis and lymphocytic exocytosis in the follicular epithelium associated with dyskeratosis, with an abundant perifollicular and perivascular lymphocytic infiltrate.58 As the lesions progress, necrotic keratinocytes coalesce to produce overall necrosis of the adjacent follicular epithelium, epidermis, and adventitial dermis. Residual fragments of hair shafts are often seen in this area of necrosis.
Erosive Pustular DermatosisErosive pustular dermatosis of the scalp is an idiopathic pustulosis without a microbial cause. It has a chronic course with multiple relapses and is characterized by the presence of pustular lesions on the scalp, along with erosions and scabs that progress to scarring alopecia.60 The histopathological findings are nonspecific,60 with epidermal abnormalities such as erosions, atrophy, acanthosis, parakeratosis, and subcorneal pustules. A nonfolliculocentric infiltrate is usually present in the dermis. This infiltrate is of mixed nature and is associated with foreign-body type giant cells. In longstanding lesions, the number of follicles is usually decreased.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Bernárdez C, Molina-Ruiz AM, Requena L. Histopatología de las alopecias. Parte II: alopecias cicatriciales. Actas Dermosifiliogr. 2015;106:260–270.




![Cicatricial alopecia in a plaque of chronic cutaneous lupus erythematosus. A, Few follicular units are observed in longitudinal sections (hematoxylin and eosin [HE] x10). B, Fibrosis around follicular remnants (HE x200). C, Same case with transversal sections (HE x20) D, Concentric fibrosis around follicular remnants (HE x200). Cicatricial alopecia in a plaque of chronic cutaneous lupus erythematosus. A, Few follicular units are observed in longitudinal sections (hematoxylin and eosin [HE] x10). B, Fibrosis around follicular remnants (HE x200). C, Same case with transversal sections (HE x20) D, Concentric fibrosis around follicular remnants (HE x200).](https://static.elsevier.es/multimedia/15782190/0000010600000004/v2_201505051007/S1578219015000529/v2_201505051007/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![Lichen planopilaris. A, Perifollicular infiltrate is observed in longitudinal sections (hematoxylin and eosin [HE] x10). B, At higher magnification, lymphocytes are observed scattered in the follicular epithelium (HE X200). C, Same case studied with transversal sections (HE x20). D, At higher magnification, perifollicular concentric fibrosis and peripheral lymphocytic infiltrate are observed (HE x200). Lichen planopilaris. A, Perifollicular infiltrate is observed in longitudinal sections (hematoxylin and eosin [HE] x10). B, At higher magnification, lymphocytes are observed scattered in the follicular epithelium (HE X200). C, Same case studied with transversal sections (HE x20). D, At higher magnification, perifollicular concentric fibrosis and peripheral lymphocytic infiltrate are observed (HE x200).](https://static.elsevier.es/multimedia/15782190/0000010600000004/v2_201505051007/S1578219015000529/v2_201505051007/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![Longstanding pseudopelade of Brocq. A, Low-magnification view showing columns of fibrosis replacing the follicles (hematoxylin and eosin [HE] x10). B, Detail of the previous image showing vertical fibrosis mixed with actinic elastosis (HE x200). C, Transversal section of the same case, showing several follicular units (HE, X20). D, At higher magnification, concentric fibrosis around follicular remnants can be observed (HE x200). Longstanding pseudopelade of Brocq. A, Low-magnification view showing columns of fibrosis replacing the follicles (hematoxylin and eosin [HE] x10). B, Detail of the previous image showing vertical fibrosis mixed with actinic elastosis (HE x200). C, Transversal section of the same case, showing several follicular units (HE, X20). D, At higher magnification, concentric fibrosis around follicular remnants can be observed (HE x200).](https://static.elsevier.es/multimedia/15782190/0000010600000004/v2_201505051007/S1578219015000529/v2_201505051007/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![Alopecia mucinosa. A, Low-magnification view (hematoxylin and eosin [HE] x10). B, At higher magnification, lakes of mucin can be observed within the hair follicles (HE x20). C, Still higher magnification view showing mucin among the keratinocytes of the follicular epithelium and a large lake of mucin (HE x200). D, Detail of the granular basophilic material within the follicular epithelium (HE x 400). Alopecia mucinosa. A, Low-magnification view (hematoxylin and eosin [HE] x10). B, At higher magnification, lakes of mucin can be observed within the hair follicles (HE x20). C, Still higher magnification view showing mucin among the keratinocytes of the follicular epithelium and a large lake of mucin (HE x200). D, Detail of the granular basophilic material within the follicular epithelium (HE x 400).](https://static.elsevier.es/multimedia/15782190/0000010600000004/v2_201505051007/S1578219015000529/v2_201505051007/en/main.assets/thumbnail/gr4.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![Folliculitis decalvans. A, Low magnification view showing several broken hair follicles with perifollicular fibrosis (hematoxylin and eosin [HE] x10). B, Detail of the previous image showing an infundibular cyst surrounded by inflammatory infiltrate and below a pigmented hair shaft surrounded by multinucleated giant cells (HE x 200). C, Transversal sections showing perifollicular fibrosis and cuffs of infiltrate around the hair follicles (HE x20). D, At higher magnification, the infiltrate can been seen to be made up of lymphocytes and plasma cells (HE x200). Folliculitis decalvans. A, Low magnification view showing several broken hair follicles with perifollicular fibrosis (hematoxylin and eosin [HE] x10). B, Detail of the previous image showing an infundibular cyst surrounded by inflammatory infiltrate and below a pigmented hair shaft surrounded by multinucleated giant cells (HE x 200). C, Transversal sections showing perifollicular fibrosis and cuffs of infiltrate around the hair follicles (HE x20). D, At higher magnification, the infiltrate can been seen to be made up of lymphocytes and plasma cells (HE x200).](https://static.elsevier.es/multimedia/15782190/0000010600000004/v2_201505051007/S1578219015000529/v2_201505051007/en/main.assets/thumbnail/gr5.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)



