The aim of the present review is to provide an update on the most important recent studies on the use of etanercept in psoriatic arthritis (PsA). Using various assessment tools, such as the Disease Activity Score 28-joint count (DAS28), the PsA Response Criteria (PsARC), and the American College of Rheumatology (ACR) score, several authors have shown that etanercept can reduce the signs and symptoms of psoriatic arthritis and inhibit radiographic progression in studies with follow-up periods of up to 2 years. There is evidence that etanercept is effective in the treatment of psoriatic enthesitis, dactylitis, and axial joint disease as well as in disease affecting the skin and nails. In clinical trials, etanercept had a safety profile similar to that of placebo and this profile did not change over time. Cost-effectiveness models have found etanercept to be the most cost-effective tumor necrosis factor inhibitor in patients with psoriatic arthritis and mild to moderate psoriasis. Etanercept has a favorable risk-benefit profile in the short term. The concomitant use of methotrexate does not alter etanercept survival.
El objetivo de la presente revisión es hacer una puesta al día sobre los trabajos más relevantes en relación con la artritis psoriásica y etanercept. El etanercept ha demostrado ser capaz de reducir los signos y síntomas de la artritis psoriásica utilizando diferentes escalas de evaluación como el DAS28, PsACR o ACR, e inhibir la progresión radiológica en estudios con seguimiento de hasta 2 años. Existen datos de eficacia en entesitis, dactilitis y afectación axial, al igual que en uñas y piel. El perfil de seguridad en los ensayos clínicos fue similar a placebo y se mantuvo en el tiempo. En modelos de coste-efectividad etanercept resulta el anti-TNF más coste-efectivo para el grupo de pacientes con artritis psoriásica y psoriasis leve o moderada. El perfil de riesgo-beneficio a corto plazo resulta favorable para etanercept. El uso de metotrexato no modifica la supervivencia del fármaco.
This review of the treatment of psoriatic arthritis with etanercept describes the general characteristics of the clinical trials with the drug and the outcome measures used. We will also discuss the mechanism of action, clinical efficacy and effectiveness, optimal dosing regimen, combination with disease-modifying antirheumatic drugs, safety and immunogenicity of the drug. Finally, the role of etanercept in the treatment of disease will be addressed in the framework of published meta-analyses and clinical equivalence and pharmacoeconomic studies.
We searched PubMed to identify publications on the efficacy and safety of the biological agents etanercept, infliximab, adalimumab, golimumab, certolizumab, ustekinumab, secukinumab, ixekizumab, and/or brodalumab in the treatment psoriatic arthritis. The search term was as follows: psoriatic, arthritis AND (etanercept OR infliximab OR adalimumab OR golimumab OR certolizumab OR ustekinumab OR secukinumab OR brodalumab OR ixekizumab) AND (randomized controlled trial OR meta-analysis). We selected randomized phase iii studies and other studies with biological agents in patients with psoriatic arthritis who had involvement of at least 2 joints.
Psoriatic Arthritis and Treatment GuidelinesPsoriatic arthritis is an immune mediated inflammatory disease with many pathophysiological and genetic susceptibility elements in common with psoriasis. The disease is classified as a spondyloarthropathy and can become manifest in the form of synovitis, enthesitis, dactylitis, and spondylitis, with different phenotypes (oligoarticular and polyarticular, axial, peripheral, and mixed) that can vary over the course of the disease. Early diagnosis is necessary to prevent the development of irreversible osteoarticular damage.1
In a recent consensus paper that used the Delphi method, some guidelines were proposed for the coordinated management of psoriatic arthritis by rheumatologists and dermatologists.2 The consensus includes therapeutic recommendations on the use of biological agents in psoriatic arthritis based on the Consensus Statement of the Spanish Society of Rheumatology (SER)3 and the recommendations of the European League Against Rheumatism (EULAR).1 There are currently 6 biological agents approved for the treatment of psoriatic arthritis: adalimumab (Humira), etanercept (Enbrel), infliximab (Remicade), golimumab (Simponi), certolizumab pegol (Cimzia), and ustekinumab (Stelara). All except golimumab and certolizumab are also approved for the treatment of psoriasis. In general, they are administered in combination with methotrexate for the treatment of psoriatic arthritis, although they can be administered as monotherapy when combination therapy with methotrexate is contraindicated. Adalimumab and etanercept are approved in monotherapy for both psoriasis and psoriatic arthritis, whereas infliximab, golimumab, and ustekinumab can be used indistinctly in monotherapy or in combination with methotrexate in the case of psoriatic arthritis.
There are no data available on head-to-head comparisons to support the superiority of one biologic over another in the treatment of psoriatic arthritis. The choice of biologic will therefore depend on the particular situation of each patient, taking into account comorbidities, prior response to other biologics, immunogenicity, route of administration, and mechanism of action of the drug.
Systematic reviews and meta-analyses have been conducted.4–8 To overcome the lack of head-to-head trials, these analyses have been used as the basis for studies of equivalence9 and economic evaluations9,10 for the treatment of psoriatic arthritis with the biologics initially available (adalimumab, etanercept, golimumab, and infliximab).
Outcome Measures for Therapeutic ResponseThe first clinical trials with etanercept in psoriatic arthritis required the development of individual and composite scales to assess the response to treatment in the different key domains of the disease.11 These domains include the joints, skin, enthesitis, dactylitis, spine, radiographically assessed joint damage, quality of life, and functional capacity.12,13 Some of these scales were borrowed from rheumatoid arthritis (American College of Rheumatology [ACR] score, Disease Activity Score [DAS]) and ankylosing spondylitis (Bath Ankylosing Spondylitis Disease Activity Index [BASDAI], Maastricht Ankylosing Spondylitis Enthesis Score [MASES]) while others (such as the Psoriatic Arthritis Response Criteria [PsARC]) had been developed specifically for psoriatic arthritis.
A specific response index was developed in a clinical trial of sulfasalazine in psoriatic arthritis.14 This subsequently became known as the PsARC and was used as the primary outcome measure in the first randomized clinical trial with etanercept.11 PsARC response is defined as an improvement in at least 2 of the 4 following measures, 1 of which should be joint tenderness or swelling score, with no worsening in any of the 4: global patient self-assessment (on a 0-5 Likert scale), global physician assessment (on a 0-5 Likert scale), improvement of at least 30% in swollen joint score, and improvement of at least 30% in tender joint score.
As a secondary response measure, the study used a modified version of the ACR20 score, which the ACR developed for rheumatoid arthritis in the pre-biologics era.15 This scale has a dichotomous outcome (presence or absence of response) based on:
- •
Improvement of 20% or more in the tender and swollen joint count
- •
Improvement of 20% or more in at least 3 of the following parameters: erythrocyte sedimentation rate (ESR) or C-reactive protein, global physician assessment of disease activity, global patient assessment of disease activity, patient assessment of pain and physical functioning
These criteria define ARC20 response and require a 20% improvement in each of the parameters. The value of 20% is considered as the cutoff for clinical relevance. ACR50 and ACR70 responses require 50% and 70% improvement, respectively, in joint counts and the above mentioned parameters.
In the trial with etanercept,14 the 8 distal interphalangeal joints of the feet and 2 carpometacarpal joints were added to the usual 68 tender joint count (2 temporomandibular, 2 sternoclavicular, 2 acromioclavicular, 2 shoulder, 2 elbow, 2 wrist, 10 metacarpophalangeal, 10 proximal interphalangeal, 8 distal interphalangeal, 2 hip, 2 knee, 2 ankle, 2 tarsal, 10 metatarsophalangeal, and 10 proximal interphalangeal joints) and 66 swollen joint count (the same joints except for the hips), yielding a total count of 78 and 76 joints, respectively.
Subsequently, the ACR 68/66 has been used systematically in clinical trials for psoriatic arthritis (infliximab, adalimumab, golimumab, etc) because of the clinical difficulties for assessing involvement in the 10 additional joints. This outcome measure has been considered stricter than the PsARC because the percentage of responders in the placebo group is lower.
Another outcome measure used in clinical trials is the DAS28, which integrates the activity score (swollen or tender) in 28 joints, ESR or C-reactive protein levels, and a visual analogue scale of disease activity for calculating the score based on a formula. Most clinical trials define the therapeutic objective as achieving DAS28<3.2, which indicates low disease activity, or DAS28<2.6, which indicates remission.
Etanercept: Findings of Clinical Trials in Psoriatic ArthritisThe efficacy of etanercept was initially demonstrated in a 12-week randomized placebo-controlled clinical trial in which 60 patients with active psoriatic arthritis (3 or more tender joints and 3 or more swollen joints) who did not respond to nonsteroid antiinflammatory drugs were assigned to receive etanercept 25mg twice a week, subcutaneously, or placebo.11 In each of the groups, the 47% of the patients who were taking methotrexate at a stable dose less than 25mg/wk continued to do so. In this 12-week study, with no open-label extension, 26 (87%) of the patients treated with etanercept achieved a PsARC response compared to 7 (23%) of those on placebo. ARC20 response was reported in 22 (73%) and 4 (13%) of patients, respectively, with no significant differences according to methotrexate treatment. By visual extrapolation of Figure 2 of the article, the ACR50 and ACR70 responses of the active treatment group were approximately 50% and 15%, respectively. In the 19 patients in each treatment group in whom it was possible to assess psoriasis (involvement of body surface area [BSA]≥3), 5 (26%) of the patients treated with etanercept achieved an increase of 75% or more with respect to the baseline Psoriasis Area and Severity Index (PASI) (that is PASI75 response) compared to no responses among patients on placebo.
In a subsequent clinical trial,16 with a 24-week placebo-controlled run-in phase and an open-label extension up to week 48, 205 patients were randomized (104 to the placebo group and 101 to the etanercept group at the same dose as the previous study). After 12 weeks, 59% of the etanercept-treated patients achieved ACR20 response compared to 15% in the placebo group (P<.0001). By visual extrapolation of Figure 2 of that article, the ACR50 and ACR70 responses of the etanercept group were approximately 40% and 15%, respectively. In all cases, the response rate among etanercept-treated patients remained stable up to week 24 of the open-label extension of the study. At 12 and 24 weeks, the PsARC response rates were 72% and 70%, respectively, for patients treated with etanercept and 31% and 23%, respectively, for those in the placebo group. Radiographic disease progression was assessed in this study using a modified total Sharp score (141 of 169 patients who entered the open-label extension phase had radiologic data available for analysis at 2 years).16 The etanercept-treated group showed inhibition at 12 months: the annualized rate of change was -0.03 units compared to +1.00 units for the placebo group (P=.0001). Inhibition was maintained during the 2-year follow-up (-0.38 with respect to baseline at 2 years, -0.22 between the first year and the second year in patients who switched from placebo to etanercept).17
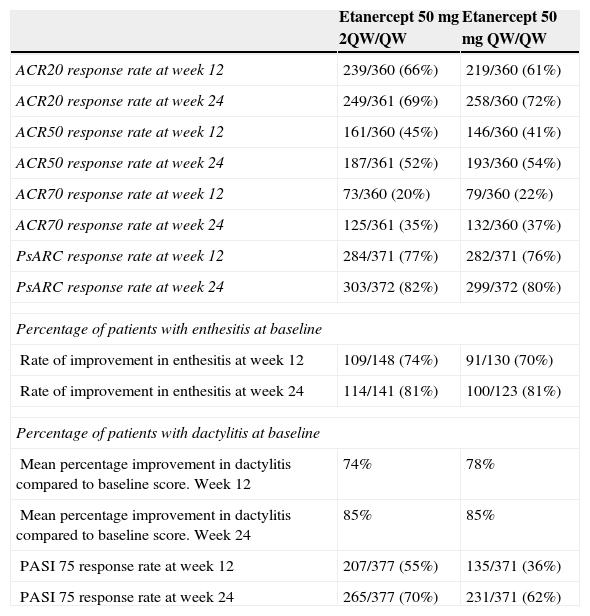
The PRESTA study included 752 patients with moderate to severe psoriasis with involvement of at least 10% of the BSA and psoriatic arthritis confirmed by a rheumatologist, with at least 2 swollen joints and at least 2 tender joints.18 However the study did not include a control group. Patients were randomized to etanercept 50mg twice a week (n=379) or 50mg once a week (n=373) for 12 weeks. They then received etanercept 50mg once a week for a further 12 weeks. The primary efficacy endpoint was achieving clear or almost clear on the physician global assessment (PGA-m) at week 12. The study also included an assessment of ACR20/ACR50/ACR70 and PsARC was well as PASI75 response, enthesitis, and dactylitis. The results are presented in Table 1. Both etanercept treatment regimens were associated with sustained and significant improvement in the quality of life of patients, and this was already apparent from the third week of treatment.19
Psoriatic Arthritis Outcomes in the PRESTA Study.
| Etanercept 50mg 2QW/QW | Etanercept 50mg QW/QW | |
|---|---|---|
| ACR20 response rate at week 12 | 239/360 (66%) | 219/360 (61%) |
| ACR20 response rate at week 24 | 249/361 (69%) | 258/360 (72%) |
| ACR50 response rate at week 12 | 161/360 (45%) | 146/360 (41%) |
| ACR50 response rate at week 24 | 187/361 (52%) | 193/360 (54%) |
| ACR70 response rate at week 12 | 73/360 (20%) | 79/360 (22%) |
| ACR70 response rate at week 24 | 125/361 (35%) | 132/360 (37%) |
| PsARC response rate at week 12 | 284/371 (77%) | 282/371 (76%) |
| PsARC response rate at week 24 | 303/372 (82%) | 299/372 (80%) |
| Percentage of patients with enthesitis at baseline | ||
| Rate of improvement in enthesitis at week 12 | 109/148 (74%) | 91/130 (70%) |
| Rate of improvement in enthesitis at week 24 | 114/141 (81%) | 100/123 (81%) |
| Percentage of patients with dactylitis at baseline | ||
| Mean percentage improvement in dactylitis compared to baseline score. Week 12 | 74% | 78% |
| Mean percentage improvement in dactylitis compared to baseline score. Week 24 | 85% | 85% |
| PASI 75 response rate at week 12 | 207/377 (55%) | 135/371 (36%) |
| PASI 75 response rate at week 24 | 265/377 (70%) | 231/371 (62%) |
Source: Adapted from Sterry et al.18
Abbreviations: 2QW, twice weekly; QW, once weekly.
The efficacy of etanercept in axial manifestations has been assessed in an multicenter observational study of 32 patients. Use of the drug was associated with an improvement in several of the outcomes for assessment of response in spondylitis20,21: response criteria were met for the Assessment of SpondyloArthritis international Society (ASAS), based on the BASDAI, in 72% of the patients. Among patients with peripheral disease, 78% and 56% of the patients in the study achieved an ARC20 and ARC50 response, respectively,
In the open-label observational study EDUCATE,22 which lasted 24 weeks and included 1122 patients with plaque psoriasis and joint involvement (2 or more tender and swollen joints for at least 3 months or 1 or more joints with sacroiliitis or spondylitis), treatment with etanercept at a dose of 50mg weekly was associated with a mean decrease of 2.7 (95% CI, 2.53-2.84) and 1.5 (95% CI, 1.39-1.55) in the scores corresponding to the patient global assessment of joint pain and joint disease, respectively.
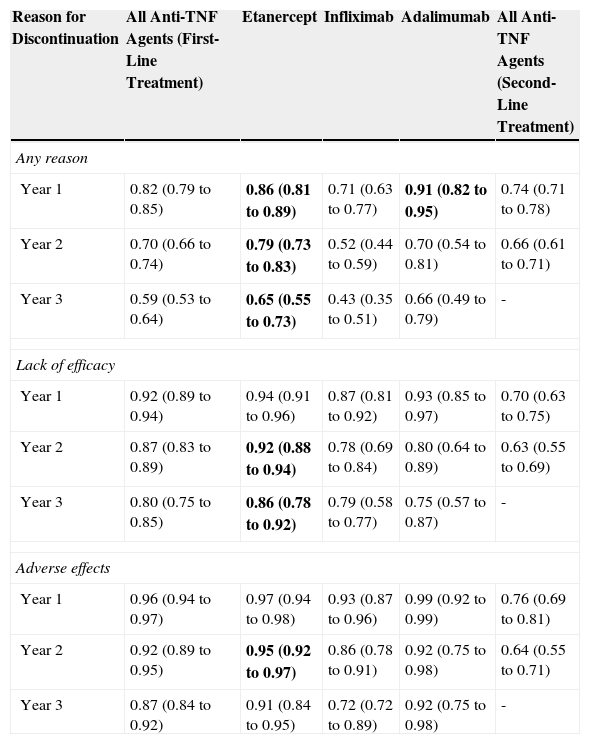
Adherence to etanercept therapy has been assessed in several observational studies and registries. In a retrospective observational study with 3 years follow-up of 650 patients with psoriasis, of whom 58.6% also had psoriatic arthritis, adherence to therapy was significantly greater with etanercept than with infliximab or adalimumab. In another retrospective observational study with 3 years follow-up in 287 patients aged 65 years or older and associated psoriatic arthritis, etanercept and adalimumab showed favorable efficacy and safety, although adherence was greater for etanercept (75.4% vs 60.7%, respectively). Data from the British Society of Rheumatology Biologics Register, including 566 patients with no prior exposure to biologics, of whom 316 (55.8%) were treated with etanercept, showed that the probability of remaining on therapy with etanercept is generally greater than with infliximab (Table 2).23 In the multivariate analysis, the hazard ratio for treatment withdrawal (all causes) with infliximab versus etanercept was 2.80 (95% CI, 2.12-3.70; P<.05). No significant differences were observed with respect to adalimumab. According to the study findings, concomitant treatment with methotrexate did not significantly alter the probability of remaining on biologic treatment, in agreement with the results of a prospective study of 82 patients with psoriatic arthritis treated with etanercept.24 In any case, the effect of combination treatment with methotrexate to improve adherence to biologics appears to be more marked in the case of infliximab.25
Survival Function for Withdrawal of First Biologic by Year of Follow-Up.
| Reason for Discontinuation | All Anti-TNF Agents (First-Line Treatment) | Etanercept | Infliximab | Adalimumab | All Anti-TNF Agents (Second-Line Treatment) |
|---|---|---|---|---|---|
| Any reason | |||||
| Year 1 | 0.82 (0.79 to 0.85) | 0.86 (0.81 to 0.89) | 0.71 (0.63 to 0.77) | 0.91 (0.82 to 0.95) | 0.74 (0.71 to 0.78) |
| Year 2 | 0.70 (0.66 to 0.74) | 0.79 (0.73 to 0.83) | 0.52 (0.44 to 0.59) | 0.70 (0.54 to 0.81) | 0.66 (0.61 to 0.71) |
| Year 3 | 0.59 (0.53 to 0.64) | 0.65 (0.55 to 0.73) | 0.43 (0.35 to 0.51) | 0.66 (0.49 to 0.79) | - |
| Lack of efficacy | |||||
| Year 1 | 0.92 (0.89 to 0.94) | 0.94 (0.91 to 0.96) | 0.87 (0.81 to 0.92) | 0.93 (0.85 to 0.97) | 0.70 (0.63 to 0.75) |
| Year 2 | 0.87 (0.83 to 0.89) | 0.92 (0.88 to 0.94) | 0.78 (0.69 to 0.84) | 0.80 (0.64 to 0.89) | 0.63 (0.55 to 0.69) |
| Year 3 | 0.80 (0.75 to 0.85) | 0.86 (0.78 to 0.92) | 0.79 (0.58 to 0.77) | 0.75 (0.57 to 0.87) | - |
| Adverse effects | |||||
| Year 1 | 0.96 (0.94 to 0.97) | 0.97 (0.94 to 0.98) | 0.93 (0.87 to 0.96) | 0.99 (0.92 to 0.99) | 0.76 (0.69 to 0.81) |
| Year 2 | 0.92 (0.89 to 0.95) | 0.95 (0.92 to 0.97) | 0.86 (0.78 to 0.91) | 0.92 (0.75 to 0.98) | 0.64 (0.55 to 0.71) |
| Year 3 | 0.87 (0.84 to 0.92) | 0.91 (0.84 to 0.95) | 0.72 (0.72 to 0.89) | 0.92 (0.75 to 0.98) | - |
Source: Saad et al.23
The survival rates for each drug whose 95% CI do not overlap with infliximab are shown in boldface. Data presented as mean (95% CI).
There are no randomized, double-blind studies to form the basis of a comparison of efficacy between the available treatments (whether biologics or not) for psoriatic arthritis. The only head-to-head comparison was an open-label study of 100 patients with psoriatic arthritis and inadequate response to previous disease-modifying antirheumatic drugs that randomized patients to infliximab 5mg/kg every 6-8 weeks (n=36), etanercept 50mg weekly (n=36), or adalimumab 40mg every 2 weeks (n=34).26 Patients maintained their previous treatment although there was a clear predominance of patients receiving treatment in combination with methotrexate in the infliximab-treated group. At 12 months, the ACR20 response rates were 75%, 72%, and 70%, respectively. Infliximab- and adalimumab-treated patients had a better response in terms of PASI, whereas those treated with etanercept had best response in terms of total joint count and physical function as measured with the Health Assessment Questionnaire.
Meta-Analyses and Clinical Equivalence StudiesThe first meta-analysis in which the different anti-TNF agents were assessed for the treatment of psoriatic arthritis included 6 trials, with a total of 982 patients.4 The ACR20 response for all anti-TNF agents was significantly greater than placebo, with a pooled relative risk (RR) of 4.35 (95% CI, 3.24-5.84). The RRs for each individual treatment were as follows: adalimumab 3.42 (95% CI, 2.08-5.63); etanercept 5.50 (95% CI, 2.15-14.04); infliximab 5.71 (95% CI, 3.53-9.25). Indirect comparisons between biologics did not show any significant differences between treatments. The same pattern was observed when PsARC response was assessed at 12 and 24 weeks. No significant differences were observed in the rates of withdrawal from the study for any reason (RR 0.48; 95% CI, 0.20-1.18) or adverse events (RR 2.14; 95% CI, 0.73-6.27), serious adverse events (RR 0.98; 95% CI, 0.55-1.77), or upper respiratory tract infections (RR 0.91; 95% CI, 0.65-1.28).
Another meta-analysis,5 also published in 2008, included 5 clinical trials with anti-TNF drugs (etanercept 2, infliximab 2, adalimumab 1) and different disease-modifying therapies. The risk of withdrawal from the study due to lack of efficacy was used as the response criteria: in the case of anti-TNF agents, the risk ratio of all the agents pooled compared to placebo was 0.25 (95% CI, 0.13-0.48; P=.0001), with the best efficacy/toxicity ratio (ratio of number needed to harm/number needed to treat=0.25). The different biologics were not assessed separately.
In a systematic review published in Health Technology Assessment in 2011, the efficacy and safety of etanercept, infliximab, and adalimumab were assessed using the data from 6 randomized, double-blind, placebo-controlled studies.6 With PsARC response as the efficacy parameter, the RRs compared to placebo were as follows: etanercept 2.60 (95% CI, 1.96-3.45), infliximab 3.44 (95% CI, 2.53-4.69), and adalimumab 2.24 (95% CI, 1.74-2.88). These results were consistent with those obtained from pooling the data for ACR20 response.
Ash et al.7 conducted a meta-analysis which formed the basis for the therapeutic recommendations of the EULAR for the treatment of psoriatic arthritis. In their analysis, the studies were pooled by therapeutic class and not by drug, although the CIs in the forest plots were superimposed for all anti-TNF agents. The RR of ACR20 response compared to placebo in the pooled analysis were 2.73 (95% CI, 2.36-3.15) for PsARC response at 12-14 weeks, 4.39 (95% CI, 3.53-5.46) for ARC20 response at 12-16 weeks, 14.61 (95% CI, 5.96-35.76) for ACR70 at 12-16 weeks, and 6.89 (95% CI, 4.64-10.23) for PASI50 response at 12-16 weeks.
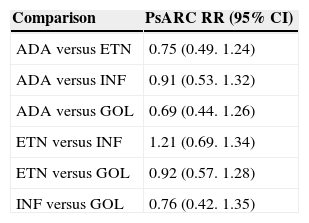
In 2012, a meta-analysis was published with the aim of directly estimating and indirectly comparing the response rates for PsARC.8 The analysis did not find significant differences between the different anti-TNF agents. The RRs compared to placebo were as follows: adalimumab 2.39 (95% CI, 1.84-3.12), etanercept 3.19 (95% CI, 2.31-4.42), infliximab 2.64 (95% CI, 1.66-4.21), and golimumab 2.45 (95% CI, 2.39-4.99). The sensitivity analysis of the outcomes at 24 weeks yielded similar results. The indirect estimates of relative risks in the binary combinations are summarized in Table 3.
Binary Comparisons of Anti-TNF Drugs by Indirect Estimation.
| Comparison | PsARC RR (95% CI) |
|---|---|
| ADA versus ETN | 0.75 (0.49. 1.24) |
| ADA versus INF | 0.91 (0.53. 1.32) |
| ADA versus GOL | 0.69 (0.44. 1.26) |
| ETN versus INF | 1.21 (0.69. 1.34) |
| ETN versus GOL | 0.92 (0.57. 1.28) |
| INF versus GOL | 0.76 (0.42. 1.35) |
Source: Thorlund et al.8
Abbreviations: ADA, adalimumab; ETN, etanercept; GOL, golimumab; INF, infliximab; PsARC: Psoriatic Arthritis Response Criteria; RR, Relative Risk.
With the 24-week ACR50 response rate as the primary efficacy measure for the indirect comparison, Fénix-Caballero et al.9 conducted indirect comparisons with the Bucher method, using infliximab as the reference and a δ of 16% (half the absolute risk reduction compared to placebo obtained in the meta-analysis) as the criterion for clinical equivalence. The estimated differences (absolute risk reduction) in terms of ACR50 response compared to infliximab were 4% (95% CI, –9.5 to 17.5) for adalimumab, 4% (95% CI, –10.5 to 18.5) for etanercept, and 9% (95% CI, –5.4 to 23.4) for golimumab. In the secondary efficacy analysis with ACR20, no significant differences were observed between drugs. However, in the analysis with ACR70, etanercept was less effective than infliximab (absolute risk reduction 17%; 95% CI, 6.2-27.8), adalimumab (absolute risk reduction 14%; 95% CI, 4.9-23.0), and golimumab (absolute risk reduction 10%; 95% CI, 1.2-18.8).
The results corresponding to the probability of achieving PsARC response from a Bayesian network meta-analysis sponsored by Pfizer and published recently10 were as follows: placebo, 0.26 (95% CI, 0.22-0.29); adalimumab, 40mg every 2 weeks 0.59 (95% CI, 0.48-0.70); infliximab 5mg/kg every 8 weeks, 0.77 (95% CI, 0.66-0.86); golimumab 50mg every 4 weeks 0.78, (95% CI, 0.66-0.87); and etanercept 25mg twice a week 0.73, (95% CI, 0.60-0.83). The sensitivity analysis yielded similar results when response was assessed at 24 weeks.
A meta-analysis of radiographic progression has been published.27 The analysis included 5 studies with 110 patients in total. After 24 weeks with anti-TNF therapy, there was no radiographic progression in 494 out of 584 patients (84.5%) compared to 362 out of 526 (68.8%) in the placebo groups, with an odds ratio of 2.68 (95% CI, 1.99-3.60; P<.001). Only 3 trials provided data on the possible efficacy of combination therapy with methotrexate; in 2 there was no significant difference and the other suggested a beneficial effect for the combination.
Economic AssessmentsA cost-effectiveness analysis sponsored by the British National Institutes of Health Research used a model based on the probability of PsARC and PASI75 response at 12-16 weeks.28 The incremental cost-effectiveness ratio in patients with psoriatic arthritis and mild to moderate psoriasis treated with etanercept compared to palliative care was approximately £18000 per Quality-Adjusted Life Year (QALY), while the ratio for infliximab compared to etanercept was £44000 per QALY, with adalimumab being the least cost-effective. For the threshold of £20000 per QALY, the calculated probability that etanercept would be cost effective was 43.6%.
The complete analysis funded by the National Institute for Health Research Health Technology Assessment program in the United Kingdom, based on published models and the cost-effectiveness analysis provided by the manufacturers, suggests that etanercept would be the most cost-effective strategy for patients with psoriatic arthritis and mild to moderate psoriasis for a threshold of £20 000-£30 000 per QALY.6 In the case of patients with psoriatic arthritis and moderate to severe psoriasis, the 3 biologics assessed (etanercept, adalimumab, and infliximab) were found to have the same probability of being cost-effective for a threshold of £20 000 per QALY.
A study published recently,10 which included golimumab and used a Markov model, with sustained PsARC response and PASI50, PASI75, and PASI90 response, with 3-month cycles, arrived at similar conclusions: using a 40-year Monte Carlo simulation, etanercept was found to be the most cost-effective treatment for a willingness to pay between £20 000 and £70 000 per QALY, and a probability of 62% for £20 000 per QALY and 70% for £30 000 per QALY.
SafetyThe safety profile of anti-TNF drugs has been extensively studied in rheumatoid arthritis and spondyloarthropathy. In a systematic review of the clinical trials with these drugs in patients with psoriasis and psoriatic arthritis (including 7 studies with a total of 1472 patients treated with etanercept), the short-term risk/benefit profile (12-24 weeks, mean of 17.8 weeks) is favorable.29 The overall odds ratios for infection and neoplasms were 1.18 (95% CI, 1.05-1.33) and 1.48 (95% CI, 0.71-3.09), respectively, with no significant differences between drugs; in the case of etanercept, the odds ratios were 1.14 (95% CI, 0.92-1.40) and 1.61 (95% CI, 0.49-5.35), respectively.
These results differ from those obtained in a meta-analysis that included trials of infliximab or adalimumab treatment in patients with rheumatoid arthritis.30 A dose-dependent increase was observed in the risk of serious infections and neoplasms in general, suggesting that there are differences in susceptibility related to the disease itself or immunosuppressive therapy (methotrexate and/or corticosteroids), which is administered much more frequently in patients with rheumatoid arthritis.30
Other reviews have investigated the safety of biological therapy with anti-TNF agents31,32 and etanercept33 in patients with psoriatic arthritis, although long-term data from disease-specific registers that taken into account use of different therapies are lacking.
ConclusionEtanercept has been shown to reduce signs and symptoms of psoriatic arthritis using different outcome measures such as the DAS28, PsARC, or ACR score. Results have suggested efficacy in enthesitis and dactylitis. The indices available for spondylitis have also shown the drug to be effective in patients with axial disease. The drug also reduces the psoriatic disease of the skin and nails and has been shown to halt radiographic disease progression in placebo-controlled studies at 24 weeks, with the effect maintained up to 2 years in extension studies. The safety profile of etanercept was similar to placebo in the blinded phase of the study and the same rate of adverse effects was observed during the 48 weeks of follow-up.
Comparison of the efficacy and safety outcomes among the different biologics available for the treatment of psoriatic arthritis requires data extracted from different meta-analyses. According to these data, etanercept is just as effective as other anti-TNF agents, except in cost-effectiveness models, where etanercept has been shown to be the most cost-effective anti-TNF drug for patients with psoriatic arthritis and mild to moderate psoriasis. In terms of safety, the short-term risk/benefit profile was favorable for etanercept.
The use of methotrexate does not significantly impact adherence to etanercept, although the exact implication of the mechanism of action of etanercept and/or the role of immunogenicity in these results is not known.
Conflicts of InterestLluís Puig received funding from Pfizer España SLU for the drafting of this manuscript.
The remaining authors declare that they have no conflicts of interest.
Please cite this article as: Puig L, López-Ferrer A, Laiz A. Etanercept en el tratamiento de la artritis psoriásica. Actas Dermosifiliogr. 2015;106:252–259.