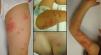
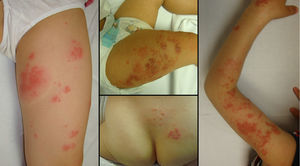
The introduction of the varicella zoster virus (VZV) vaccine was expected to eliminate herpes zoster (HZ) in vaccinated children. However, several years later we continue to treat cases of HZ in children. We describe 8 cases of pediatric HZ that were recorded between 2010 and 2013 during duty shifts at the dermatology service of Hospital Universitario Fundación Alcorcón. During this period the population living in the catchment area of the hospital corresponded to 247 000 inhabitants. The clinical and epidemiological characteristics of the patients are shown in Table 1 and Figure 1. A slight male predominance was observed, with a male to female ratio of 6:2. Ages ranged from 18 months to 5 years (mean, 3.5 years). All the patients had been vaccinated with a single dose between 15 and 17 months of age. The mean time from vaccination to HZ onset was 2.2 years, with the earliest case detected only 3 months after vaccination. Polymerase chain reaction (PCR) for VZV was performed in 2 cases, both of which were positive. The necessary technique for differentiation between the vaccine and wild strain was unavailable in our hospital. The most frequently affected dermatomes were those of the lower limbs. Treatment varied depending on the extent and duration of the lesions, and the discomfort reported by the patients. Treatment with oral acyclovir was required in 5 of the 8 cases; the remaining patients received topical treatments consisting of astringent soaks and antibiotic ointments. None of the patients had risk factors for immunosuppression or associated comorbidities. Just 3 patients had atopic dermatitis, but none of them had required oral corticosteroid therapy in the preceding year. Six of the 8 patients had undergone laboratory testing in the preceding month, with no abnormalities detected. A favorable clinical course was observed with the prescribed treatments. Subsequent follow-up revealed no complications such as scarring and postherpetic neuralgia or recurrences.
Clinical and Epidemiological Characteristics of Patients.
| Patient | Sex | AD/Asthma | Immunosuppression | Hemogram | Age at Vaccination, mo | →→ | Age, mo | Site | PCR | Second Episode | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Girl | AD | No | – | 16 | 43 | 59 | Thigh | No | No | Topical |
| 2 | Boy | No | No | Normal | 15 | 7 | 22 | Thigh | + | No | Oral acyclovir |
| 3 | Boy | No | No | Normal | 15 | 56 | 71 | Chest | No | No | Oral acyclovir |
| 4 | Boy | No | No | Normal | 15 | 29 | 44 | Face | + | No | Oral acyclovir |
| 5 | Boy | No | No | - | 17 | 20 | 37 | Ankle | No | No | Oral acyclovir |
| 6 | Girl | DA | No | Normal | 15 | 14 | 29 | Thigh | No | No | Topical |
| 7 | Boy | DA | No | Normal | 15 | 3 | 18 | Thigh | No | No | Oral acyclovir |
| 8 | Boy | No | No | Normal | 15 | 48 | 63 | Arm | No | No | Topical |
Abbreviations: →→, months from vaccination to onset of herpes zoster infection; AD, atopic dermatitis; PCR, polymerase chain reaction.
The VZV vaccine is a live attenuated vaccine derived from the Oka strain of VZV. Its use in children under 12 years was approved in 2003 (Varivax). Although the latest guidelines of the Spanish Association of Pediatrics recommend administering 2 doses (the first at 12-15 months and the second at 2-3 years), this dosing schedule is used by only 3 autonomous communities (Ceuta, Melilla, and Navarra). The general trend is to administer a single dose at 11 to 12 years. Between 2010 and 2013 children in Madrid received a single dose at 15 months, and vaccination was recommended for children aged 11 who were seronegative and had not been previously vaccinated. These recommendations were recently changed, and as of January 1, 2014, vaccination is only recommended for 12-year-olds who have not been previously vaccinated and have not contracted HZ.
A total absence of HZ in vaccinated children was expected with the introduction of the VZV vaccine, but several years later HZ cases continue to be recorded in both vaccinated and healthy children. PCR was performed in some of these cases, and was positive for the Oka strain.1–4 This strain may be reactivated, causing disseminated zoster (which occurs if antibody titers are low, and is often mistaken for wild-type infection) or metameric zoster.3 The latter condition is probably underdiagnosed owing to the belief that the vaccine strain is incapable of reactivating, the rarity of HZ in children, and the fact that postvaccination HZ is relatively mild and thus may account for fewer consultations.3 Reactivation may be more frequent if titers of anti-VZV are low3 and if a rash develops after vaccination, as it is postulated that skin lesions enable the passage of VZV to the nerves and the establishment of latent infection.2,5 Postvaccination HZ is distinguished from HZ after primary infection mainly based on the associated lesions. Postvaccination HZ lesions are generally smaller, less painful, and contain fewer vesicles. Moreover, these lesions predominantly develop on lumbosacral rather than thoracic dermatomes, given the greater proximity of the former to the site of vaccine administration.6
In conclusion, we have presented 8 cases of HZ; all patients were under 5 years of age, healthy, and had been vaccinated for VZV. Although cases of reactivation of the Oka strain of VZV have been reported, recent studies found no increase in the incidence of HZ in vaccinated children.7 However, no epidemiological studies have assessed the true incidence of HZ in children since the introduction of routine vaccination in Spain. Molecular characterization of the virus could provide more information on the incidence of HZ after VZV vaccination.
Please cite this article as: Caro-Gutiérrez D, López-Estebaranz J, Naz-Villalba E, Ayala-Bernaldo de Quiros L. Herpes zóster en niños vacunados contra el virus varicela zóster: experiencia en nuestro hospital. Actas Dermosifiliogr. 2015;106:329–331.