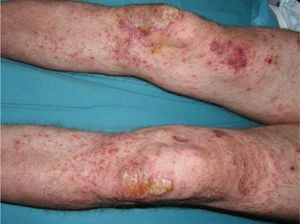
Despite the efficacy of tumor necrosis factor (TNF) α inhibitors in the management of moderate to severe psoriasis, some adverse effects associated with psoriasis have been reported in patients undergoing treatment with these biologic agents. The most frequently described effects are new-onset psoriasis in patients with no history of the disease and exacerbation or modification of the morphology of a previously diagnosed psoriasis. A large percentage of new-onset psoriasis is in the form of pustular psoriasis, mainly affecting the palms and soles,1,2 whereas guttate psoriasis is more common in patients with a prior history of the disease.3,4 We describe a patient with plaque psoriasis that was being treated with etanercept, who presented an exacerbation due to a change in the morphology of the disease to generalized pustular psoriasis, immediately after undergoing a purified protein derivative (PPD) tuberculin skin test, suggesting an association between the two events.
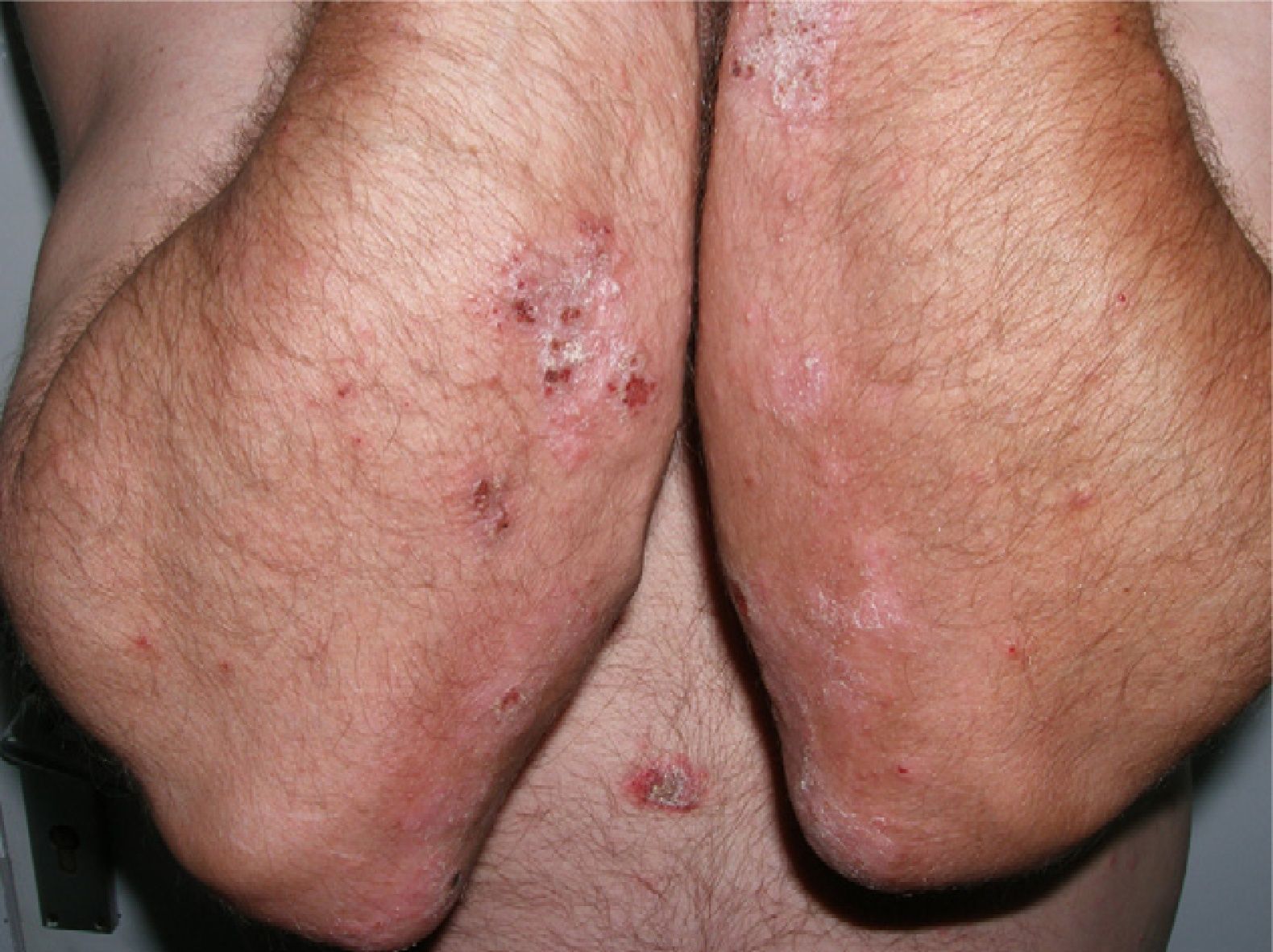
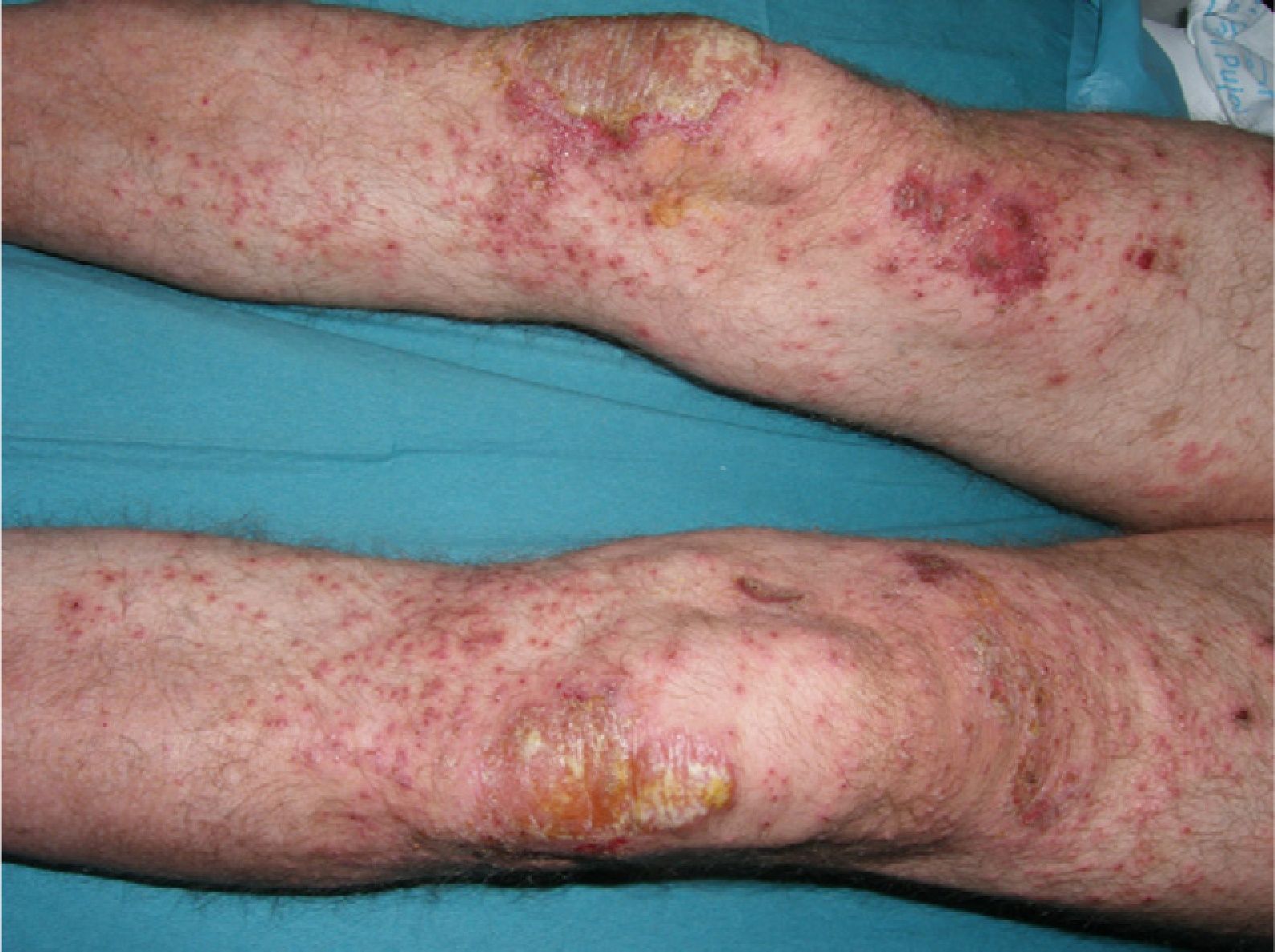
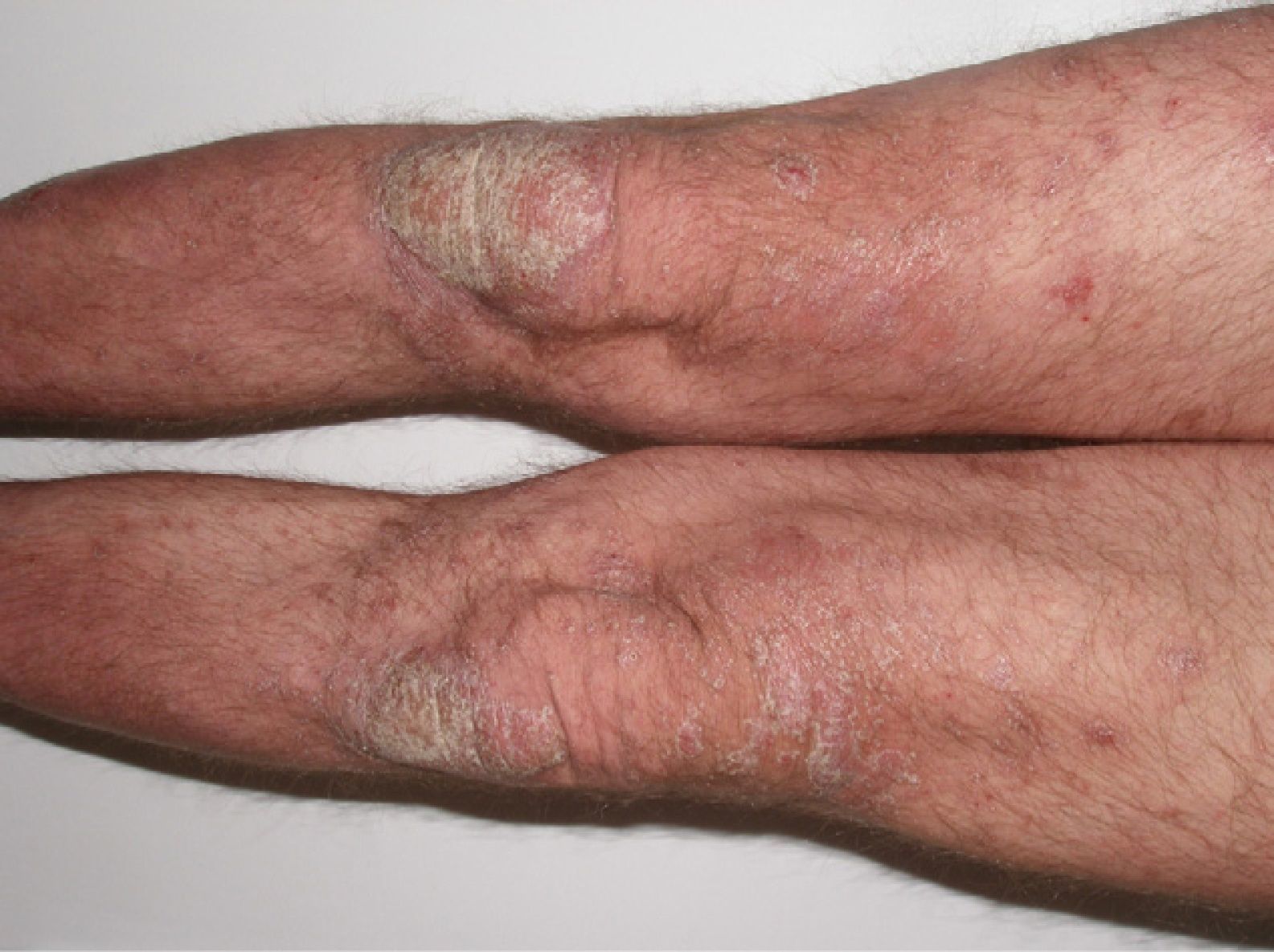
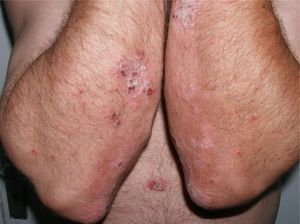
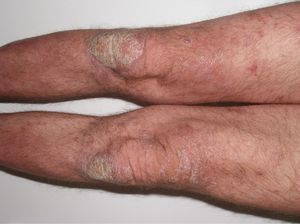
The patient was a 49-year-old man with a 29-year history of plaque psoriasis, which had previously been treated with topical corticosteroids; he had undergone 2 years of treatment with acitretin until the therapy was stopped due to dyslipemia. In 2005, with a psoriasis area and severity (PASI) score of 19.8, the patient began treatment with etanercept (25mg twice a week), with an excellent response in the first year. Four months after stopping treatment, the patient suffered a relapse (PASI score, 22) and it was therefore decided to reinstate treatment with etanercept at a dosage of 50mg twice a week for the first 12 weeks, with a subsequent maintenance dosage of 25mg twice a week. The patient's condition was adequately controlled during the initial 3 years of treatment, with a mean PASI score of 3. However, 24hours after undergoing the routine annual PPD test, pustules developed on the residual psoriasis plaques (Fig. 1); 48hours later, the rash had evolved into generalized pustular psoriasis, mainly involving the extremities (Fig. 2). Bacterial cultures were negative and histology was compatible with a diagnosis of pustular psoriasis. Treatment with etanercept was suspended due to a suspected paradoxical reaction with the drug, and the patient was started on ciclosporin (4mg/kg/d). The episode was brought under control after 2 weeks (Fig. 3). Treatment was then started with ustekinumab, while the dosage of ciclosporin was gradually reduced.
Psoriasis may be paradoxically exacerbated or induced by the 3 available anti-TNF agents.2,5 However, etanercept is more usually associated with exacerbations of previously existing psoriasis, whereas monoclonal antibodies (infliximab and adalimumab) mainly induce an initial outbreak of psoriasis.6 The mechanisms that cause these paradoxical effects are unknown. No trigger factors have been identified in pustular psoriasis of the palms and soles and a potential causal role of TNF-α inhibitors has been suggested. Generalized pustular psoriasis has been associated with factors such as pregnancy, infections, severe skin irritation caused by topical medication, and some drugs (lithium, salicylates, indomethacin, and various β-blockers). On the other hand, generalized pustular rashes in patients with stable plaque psoriasis have been linked to ultraviolet light, infections, and allergic contact dermatitis,7,8 but have not been reported in the literature in patients with psoriasis being treated with anti-TNF-α agents. In our patient, the acute outbreak only 24hours after performing the PPD skin test and the continuous blanching achieved in the previous 3 years of treatment with etanercept suggests a synergistic effect of the combination of the biologic agent and the diagnostic test. Generalized pustular psoriasis as a complication of PPD is not reported in the literature, but a case has been described of a patient with a history of plaque psoriasis, who was receiving no systemic treatment when the tuberculin skin test was performed; the patient presented a pustular exacerbation of the psoriasis after the skin test was performed.9 The pustular episode in our patient could also be considered as an atypical presentation of acute exanthematous pustulosis due to the tuberculin skin test. However, the histology findings and the coexistence of some typical plaque psoriasis lesions with the pustular rash make this option unlikely. Thus, although the chronologic course is compatible with a direct causal link between the PPD skin test and the episode, the explanation for this phenomenon continues to be a subject of speculation. The tuberculin test may have acted as a traumatic or infectious trigger that activated the patient's innate immunity, thus increasing TNF-α production by the plasmacytoid dendritic cells and stimulating local activation and proliferation of pathogenic T cells, thereby leading to the episode. Because treatment with the same agent (or an agent of the same family)5 could have caused the episode to persist, it was decided to change to a treatment with a different mechanism of action; the lesions were brought under control in a few days. In conclusion, our case extends the tuberculin-related complications that may be seen in patients with psoriasis who are undergoing treatment with etanercept.
Conflicts of InterestDr. Carlos Ferrándiz and Dr. José Manuel Carrascosa have received fees as consultants and/or speakers sponsored by Wyeth, Abbot, Schering-Plough, and Janssen-Cilag.
The other authors declare that they have no conflicts of interest.
Please cite this article as: Guinovart RM, et al. Brote de psoriasis pustulosa después de la prueba de la tuberculina en un paciente con psoriasis en plcas en tratamiento con etanercep. Actas Dermosifiliogr.2011;102:828-830.