Alopecia areata (AA) is an autoimmune disorder causing nonscarring hair loss. It is an organ-specific response at the cellular level and is mediated by CD4+ and CD8+ T cells. These lymphocytes may be activated by autoantigens at the hair root, inducing inflammation that eventually leads to hair loss. The increased use of biologic therapies has recently led to a growing number of publications discussing the possible link between these treatments and autoimmune disorders, including AA.
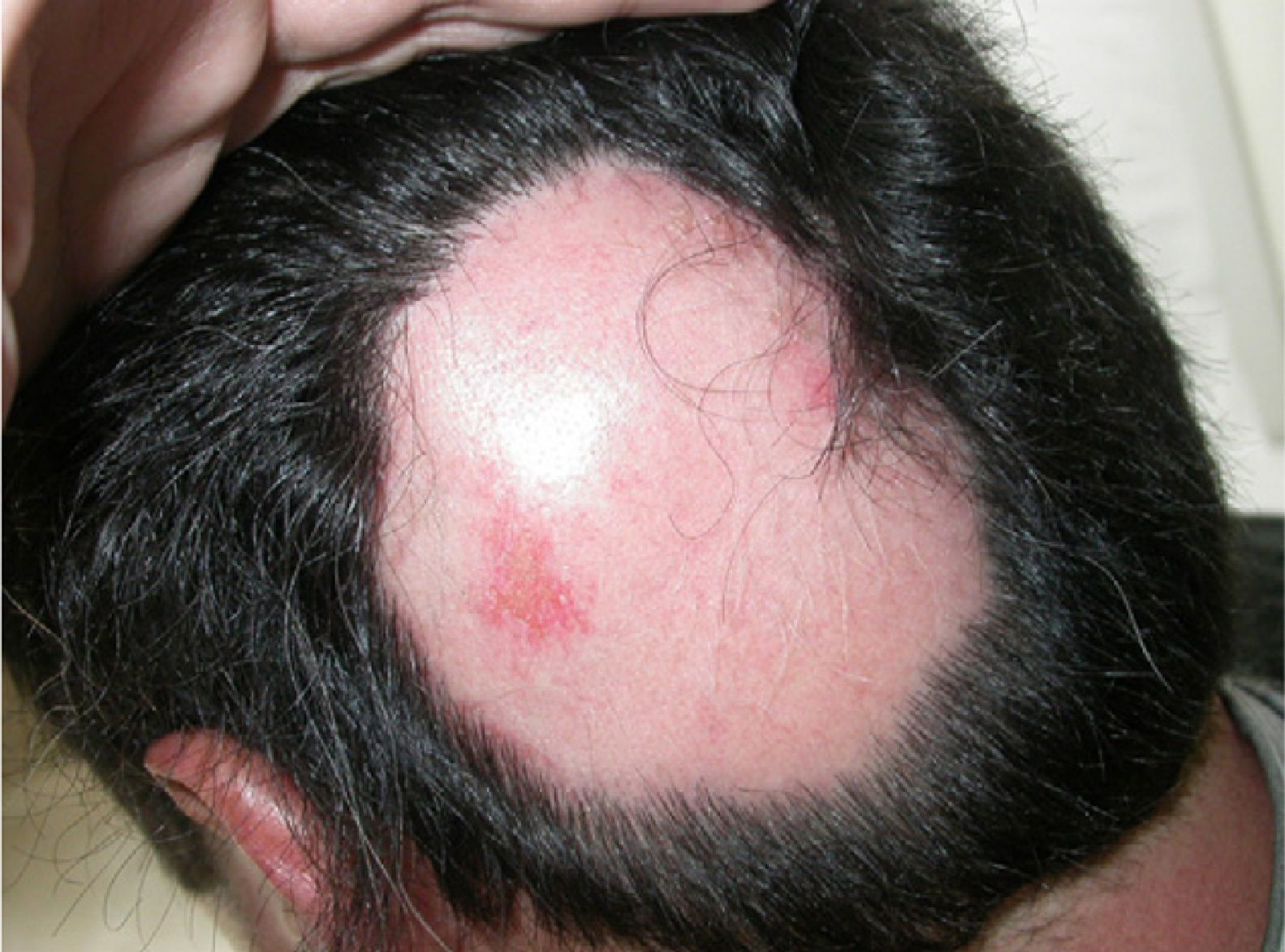
A 39-year-old man with plaque psoriasis on a regimen of adalimumab (40mg) twice weekly presented with 2 alopecia plaques after 1 year of treatment. The plaques, located on the cranial vertex, had appeared over the previous few weeks (Fig. 1). The patient had no family or personal history of AA and reported no events involving infection, vaccination, or stress prior to the loss of hair. AA was diagnosed, adalimumab treatment was stopped, and a daily application of clobetasol propionate 0.05% cream was prescribed. After 6 months the diameter of both alopecia plaques had not changed despite the withdrawal of adalimumab.
Adalimumab is a recombinant and fully humanized monoclonal antibody that works against tumor necrosis factor α (TNF-α) and has been approved for the treatment of rheumatoid arthritis, ankylosing spondylitis, Crohn disease, psoriasis, and psoriatic arthritis. TNF-α is a proinflammatory cytokine in the body's immune system, but when it is neutralized by anti-TNF-α antibodies like adalimumab, autoimmune disorders such as systematic sclerosis and systemic lupus erythematosus have been known to develop.1 The role of TNF-α in the physiopathology of these processes is complex, but the cytokine has been shown to originate in the mononuclear cells that surround the hair follicle in AA1 and inhibit hair follicle growth during in vitro testing.2 TNF-α is thus linked to hair loss. Accordingly, anti-TNF-α medications and other biologic therapies have been used to treat AA, but with poor results; in some sporadic cases satisfactory responses have been reported, but no large study has demonstrated any significant therapeutic effects of etanercept,3 alefacept,4 or efalizumab.5
At the same time, new cases of AA have become more frequent in patients undergoing treatment with anti-TNF-α and other biologic therapies. Cases of AA induced by etanercept, infliximab, and adalimumab are being reported. García Bartels et al6 were the first to describe adalimumab-induced AA; the patient was a 23-year-old woman who developed AA universalis 2 months after starting treatment. Seven patients have since been described; only 2 had a past history of AA and 3 developed the universalis form.1,6–10 The amount of time from starting treatment with anti-TNF-α to the appearance of AA ranged between 2 months and 2 years. In cases in which adalimumab was withdrawn, no new hair growth occurred in any of the patients studied. Similarly, hair did not regrow on our patient's plaques.
The mechanism by which TNF-α inhibition induces AA is unknown. In 2005, De Bandt et al11 hypothesized, on the basis of 22 cases of anti-TNF-α-induced systemic lupus erythematosus in France, that TNF-α initiates the spread of suppressed CD4+ regulatory T lymphocytes that are responsible for maintaining immunological tolerance and preventing autoimmunity. Thus, both AA and other autoimmune processes induced by anti-TNF-α may develop through the inhibition of regulatory T cells. However, whereas all patients with lupus went into remission after the withdrawal of anti-TNF-α, patients with AA do not usually respond.
In this new case of AA associated with adalimumab treatment, the possibility of coincidence cannot be ruled out; however, earlier reports of AA in patients treated with adalimumab and the implication of anti-TNF-α medications in inducing other autoimmune disorders suggests an association between hair loss and the use of this TNF inhibitor.
Please cite this article as: Neila J, et al. Alopecia areata y terapias biológicas. Presentación de un caso asociado a adalimumab. Actas Dermosifiliograf.2011;102:827-828.