Eccrine porocarcinoma (EPC) is the most common malignant neoplasm of the eccrine gland and originates in the ductal portion of the gland.1 It was described by Pinkus and Mehregan in 1963.2 It is a rare tumor that accounts for 0.005% to 0.01% of all skin tumors.3 While most arise de novo, in 18% to 50% of published cases EPC developed in parallel with a pre-existing eccrine poroma.4 EPC usually occurs in individuals over 50 years of age and affects males and females equally.5 It most frequently develops in the lower extremities, head, and neck. The clinical presentation of EPC is varied, and can simulate that of other skin tumors. Chronic sun exposure, exposure to chemical agents, and immune suppression have been proposed as predisposing factors.5 The local recurrence rate after surgical treatment is 20%, the regional lymph node metastasis rate is 20%, and the distant metastasis rate is 11%.6
A retrospective, descriptive study of EPC cases diagnosed between 2014 and 2018 was carried out at the University Hospital Complex in León, Spain. For each patient, clinical histories and biopsy sheets were reviewed to characterize epidemiology, clinical course, poor histological prognostic factors, and immunohistochemistry findings. Descriptive statistics were used to present the data.
Eleven cases of EPC were identified (5 men and 6 women). The corresponding clinical and histological characteristics are shown in Table 1. The median (range) age of presentation was 83 (53–91) years.
Eccrine Porocarcinoma Cases: Clinical and Histological Characteristics, Treatment, and Course
| Case No. | Age, y | Sex | Location | Clinical Characteristics | Initial Lesion Size, cm | Time Since Onset, m | History of Diseases of Interest | Immunohistochemistry | Growth Pattern | Tumor Thickness, mm | Lymphovascular Invasion | Mitoses per High-Power Field | Presumptive Diagnosis | Treatment | Course (Follow-up) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 91 | F | Right pectoral | Erythematous infiltrated plaque | 4 | ND | AK | CEA, EMA, CK19 (+) | Infiltrative–pagetoid | 5.4 | Yes | 5 | SCC | Biopsy of previously excised lesions | Died after 5 days due to decompensation of comorbidities |
| 2 | 77 | F | Right leg | Grayish hyperkeratotic erythematous plaque | 1 | ND | SCC | Not performed | Expansive with pagetoid foci | 2.13 | None | 2 | SCC | Conventional surgery | No recurrence (4 y 6 m) |
| 3 | 83 | M | Left thumb | Brownish hyperkeratotic excrescent plaque | 2.1 | 12 | SCC | CEA, EMA (+) | Expansive with foci of infiltration | 2.78 | None | 2 | SCC | Conventional surgery | No recurrence (3 y 10 m) |
| 4 | 72 | F | Interparietal | Grayish hyperkeratotic plaque | 5 | 6 | Melanoma in situ, AK, BCC | CEA, EMA, CK AE1/AE3 (+) | Expansive | 7.5 | Yes | 23 | SCC | Conventional surgery | No recurrence (1 y 7 m) |
| 5 | 87 | F | Right cheek | Erythematous plaque | 3.5 | 48 | BCC, SCC | Not performed | Expansive with foci of infiltration | 3.13 | No | 3 | SCC, Bowen | Conventional surgery | No recurrence (1 y) |
| 6 | 89 | F | Right thigh | Erythematous–violaceous keratotic plaque | 1 | 4 | AK | EMA, CK19, CK AE1/AE3 (+) | Minimally infiltrative with pagetoid permeation | 2.5 | No | 2 | SCC | Conventional surgery | No recurrence (8 m) |
| 7 | 75 | F | Left thigh | Eroded keratotic plaque | 1 | 2 | AK | CEA, EMA, CK19 (+) | Focally infiltrative with some pagetoid permeation | 3.88 | No | 5 | Porocarcinoma, SCC, BCC, melanoma | Conventional surgery + widening.5 cm + inguinal ultrasound and FNA of ganglion (-) | No recurrence (6 m) |
| 8 | 85 | M | Interparietal | Crusted ulcer | 1.5 | 48 | AK | Not performed | Expansive with pagetoid foci | 1.75 | None | 1 | SCC | Conventional surgery | No recurrence (3 m) |
| 9 | 76 | M | Pubis | Ulcerated papillary plaque | 5 | ND | Non-Hodgkin lymphoma | CEA, EMA, CK19 (+) | Infiltrative | 10.38 | Yes | 3 | SCC | Conventional surgery + bilateral inguinal lymphadenectomy + chemotherapy + radiotherapy | Died after 10 m due to pulmonary metastases from EPC |
| 10 | 83 | M | Fourth finger | Reddish excrescent polyp | 3 | 6 | None | CEA, EMA, CK19 (+) | Infiltrative | 8.15 | Yes | 2 | Pyogenic granuloma, melanoma, SCC, Merkel cell | Mohs surgery + axillary ultrasound-) | No recurrence (6 m) |
| 11 | 53 | M | Left cheek | Erythematous violaceous ulcer with pearly borders | 1.5 | 12 | AK | CEA, EMA, CK19 (+) | Expansive with pagetoid foci | 3.83 | None | 1 | BCC | Conventional surgery | No recurrence (4 y 6 m) |
Abbreviations: AK, actinic keratosis; BCC, basal cell carcinoma; EPC, eccrine porocarcinoma; F, female; FNA, fine-needle aspiration; M, male; ND, not determined; SCC, squamous cell carcinoma.
High-risk data are highlighted in bold.
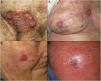
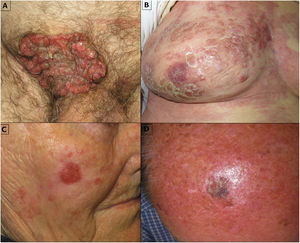
There was a history of skin tumors in 81% of patients, as follows: actinic keratosis, n = 9; basal cell carcinoma, n = 2; squamous cell carcinoma (SCC), n = 3; melanoma, n = 1. The most commonly affected locations were the lower extremities and the head. The median (range) time since onset was 17.2 (2–48) months. Clinical presentation varied (Fig. 1A–D), although the most common presentation was a hyperkeratotic erythematous plaque.
The most frequent suspected diagnosis was SCC. Differential diagnoses included pyogenic granuloma, melanoma, and basal cell carcinoma.
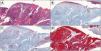
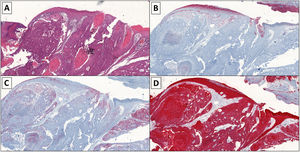
Histopathology revealed a clear infiltrative pattern in 3 cases. Median tumor thickness was 4.68 mm. In 1 case, more than 14 mitoses per high-power field were detected. Additional immunohistochemical tests (carcinoembryonic antigen [CEA], epithelial membrane antigen [EMA], and cytokeratin [AE1/AE3]) were required in 8 cases (Table 1, Fig. 2A–D).
A, Histology image showing large cells with vesicular nuclei, a visible nucleolus and ample cytoplasm, cellular atypia, and numerous atypical mitoses (hematoxylin-eosin, original magnification ×4). The cells are arranged in trabeculae, cords, and nests and areas of tumor necrosis are visible. B, Immunohistochemistry image showing weak positivity (carcinoembryonic antigen, original magnification ×4). C, Immunohistochemistry image showing positive epithelial membrane antigen staining (original magnification ×4). D, Immunohistochemistry image showing positive cytokeratin (AE1/AE3) staining (original magnification ×4).
Four patients met the criteria for high-risk EPC (Patients 1, 4, 9, and 10 in Table 1). At the moment of consultation in our center, Patient 1 had extensive skin metastases (Fig. 1B) adjacent to the scar left after excision of an EPC 1 month earlier in another center. Due to her age and comorbidities, the patient refused an analysis of tumor extension and additional treatment, and died in less than 1 week due to decompensation of comorbidities. Patient 9 presented a large ulcerated tumor on the pubis (Fig. 1A). An initial biopsy was compatible with SCC. The patient underwent wide tumor excision and bilateral inguinal lymphadenectomy. Two months later, he presented with metastasis of EPC on the surgical scar, subsequently developed lung metastases and, despite treatment, died. Patient 10 underwent Mohs surgery and had a negative sentinel lymph node biopsy (SLNB). Patient 4 was treated with conventional surgery, and has had no recurrences to date.
Patients with localized tumors were treated with conventional surgery. No recurrences were recorded after a median (range) follow-up of 22.6 (3–53) months.
The clinical and histological characteristics in our series are similar to those previously reported,7 except for median age, which was higher in our study and outside the range reported in the literature (median [range], 65 [50–80] y). A definitive diagnosis was established by standard histology and compatible immunohistochemical staining8 (Fig. 2A–D).
In addition to helping establish a definitive diagnosis, histology provides prognostic information that can facilitate patient management. Robson et al reported that infiltrative and/or pagetoid growth patterns were associated with an increased risk of local recurrence. Tumors thicker than 7 mm, an elevated mitosis count (>14 mitoses per high-power field), and the presence of lymphovascular invasion have been associated with an increased risk of distant metastasis.9 Some authors, including Belin et al, propose Mohs surgery for tumors with infiltrative and/or pagetoid patterns.10 Others propose SLNB for high-risk tumors.6 However, there is little evidence of the value of this approach owing to the paucity of documented cases.
Although a rare tumor, an increase in the incidence of EPC is likely given the growing elderly population. Early diagnosis and treatment of EPC is important owing to this tumor’s tendency to recur locally and its metastatic potential. When proposing treatment protocols, it may be necessary to establish a series of clinical and histological prognostic factors that define high-risk EPC, similar to those that exist for other tumors such as SCC.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Olmos Nieva CC, Samaniego González E, González Morán MA, Rodríguez Prieto MA. Porocarcinoma ecrino: estudio clínico-histológico de una serie de 11 casos del Complejo Asistencial Universitario de León. Actas Dermosifiliogr. 2021;112:478–481.