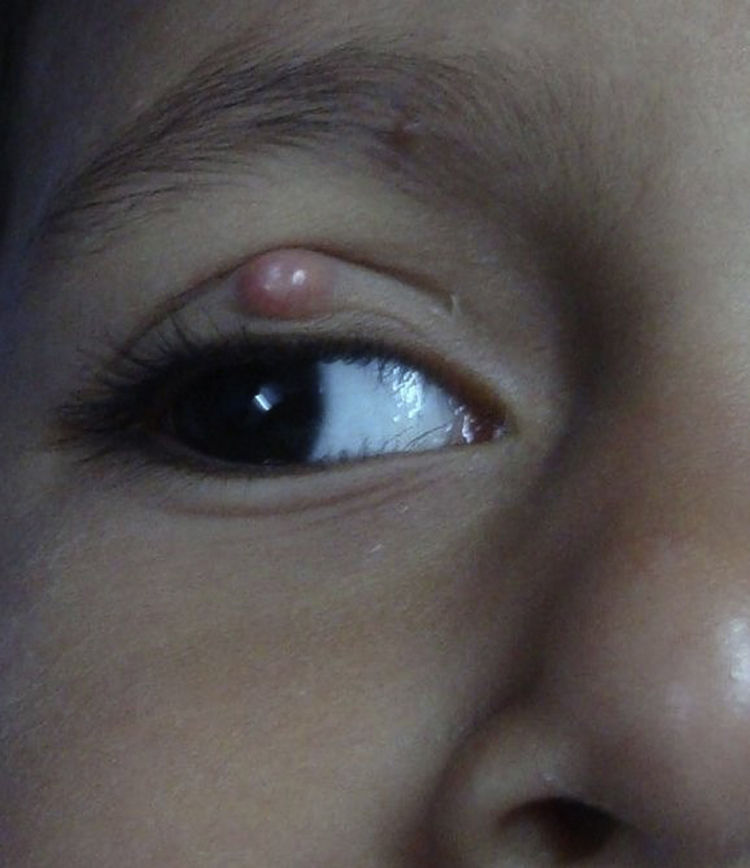
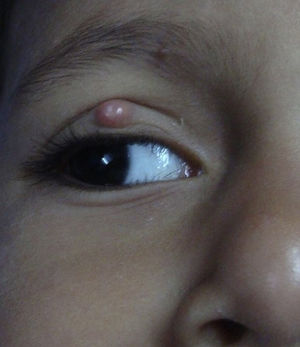
Molluscum contagiosum (MC) is a cutaneous viral infection caused by the molluscum contagiosum virus which is commonly observed in children and immunocompromised patients. It presents as single or multiple, discrete white or flesh-colored papules of sizes 1-5 mm in diameter and with typical central umbilication.1 Various treatment modalities available to treat MC include curettage, cryotherapy, extraction by a needle, and topical vesicants such as cantharidin, potassium hydroxide, salicylic acid, retinoids, silver nitrate and phenols. Immunotherapy with imiquimod, nitric oxide, and cimetidine have also been used with variable efficacy.1,2
Cantharidin is a terpenoid derived from the bodies of blister beetle which induces blistering through acantholysis and heals without scarring; hence its efficacy in MC lesions.2 However, there is a paucity of data on its use for facial MC because current convention discourages use of cantharidin on the face.3
We performed a prospective open-labeled uncontrolled interventional study to evaluate the efficacy of cantharidin in treatment naive facial MC in children of ≤10 years. Patients with secondary bacterial infections, underlying immunodeficiencies and known hypersensitivity to cantharidin were excluded. Investigations performed in all patients included complete blood count, blood sugar, urine analysis and HIV antibodies. Parents were counseled and written consent was obtained.
Each lesion was treated with cantharidin 0.7% solution (CANTHACUR® Paladin labs Inc.) with a cotton-tipped applicator. Patients were instructed to wash the treated areas only after 4 hours. Levocetirizine syrup and mupirocin ointment were prescribed for 5 days. Repeat application was performed every 2 weeks of interval for a maximum of 5 doses. Participants and/or their parents were advised about hand hygiene, avoidance of shared personal clothing or items and cessation of scratching.
At each visit, the clinical response and adverse events were assessed. The number of lesions at the beginning and at the end of the treatment was counted. The response at the end of the treatment was classified as complete (complete clearing-off lesion and appearance of no new lesions), moderate (clearing-off all treated lesions but persistence or occurrence of new lesions), mild (clearing of few but not all of the treated lesions and persistence of new lesions), and none (no response).
Differences between means of the lesions before and after the treatment were compared by student paired t test. Statistical analysis was done using Graphpad Prism 7 software.
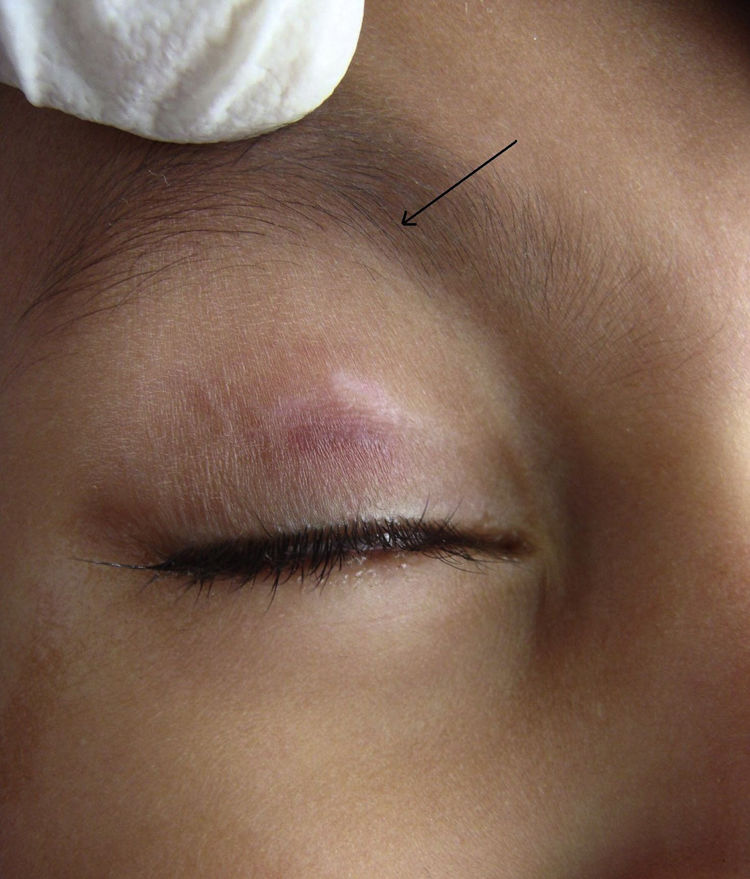
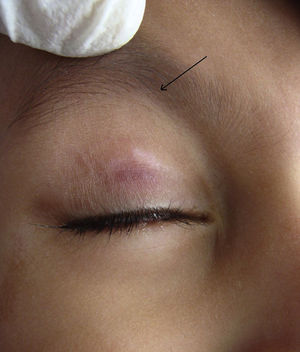
During the six recruiting months, 71 patients attending our care fulfilled the inclusion criteria and did not exhibit any of the exclusion criteria. Sixty-seven patients consented to participate in the study, out of which four were lost to follow-up. Upon telephonic conversation with each of them, it was confirmed that their drop-outs from the study were unrelated to adverse events caused by a cantharidin. Therefore, the studied population comprised a total of 63 patients, 45 males and 18 (24%) females, with a median age of 6 years (range 1-10). The median duration of disease was 4 months (range 1-10 months). Sixty (95%) patients, showed a complete response. In these patients the lesions completely healed with the formation of crusts, which then shaded off within one week (Fig. 1 and 2). One patient each showed a moderate response, and a mild response; whereas 1 patient did not respond. The mean lesion count at a baseline was 11.7 ± 4.3 and after 5 doses (5 weeks) was 1.9 ± 0.7 (p < 0.001).
As expected, 56 (90%) of the treated children experienced blistering in the treated areas which ruptured within 3-4 days and healed with uneventful recovery. Ten patients complained of mild to moderate degree of pain. Three patients developed secondary bacterial infection at the treated sites requiring systemic and topical antibiotic for short duration. Two patients developed hypo/depigmentation at the treated site which healed without any sequelae.
We found a good acceptance of cantharidin therapy and that patient’s anxiety at follow-up visits was minimal. Interestingly, we did not observe any residual scars after treatment with cantharidin, which is the most fearful outcome of cantharidin use over face.
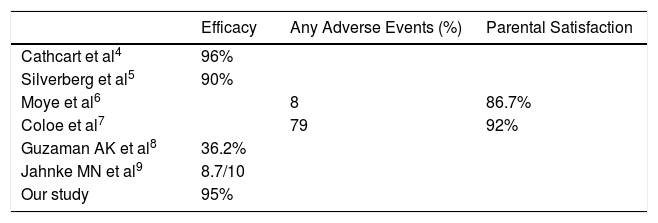
Due to its efficacy and tolerability due to painless nature, cantharidin has produced satisfaction with its use ranging from 60-90% in parents and 92% among the dermatologists.3 There are many studies describing the efficacy of cantharidin in MC but of non-facial regions. Till date only one retrospective study has been conducted to evaluate the efficacy of cantharidin in MC involving only face with good results (Table 1).
Advantages of cantharidin include quick action, lack of pain at the time of application, and since there is no trauma, the incidence of bleeding is nil. This merits the patient subsequent follow-up office visits and treatment. The limitations in our study include the small sample size, the absence of randomization, and lack of control.
In conclusion, cantharidin, when applied in a proper and controlled fashion combined with adequate patient and parents’ education, is a safe and effective therapy for childhood MC involving the face.
Conflicts of interestThe authors have nothing to disclose.
Please cite this article as: Zawar V, Pawar M, Singh M. Eficacia del tratamiento con cantaridina del molusco contagioso facial en niños pequeños: un estudio prospectivo intervencionista en 67 niños. Actas Dermosifiliogr. 2021;112:481–483.