The main symptom of atopic dermatitis (AD) is itching, which can affect the patients’ quality of life, preventing a good night sleep and negatively affecting the patients’ work or academic productivity.1
The main aim of this study was to assess the early safety, efficacy, and improvement of quality of life of dupilumab in adult patients with severe AD within the first 4 weeks of treatment.
We conducted a prospective study including all patients who started dupilumab from February 2020 through January 2021 in a single center in Spain. The inclusion criteria were: patients older than 18 years with a baseline Eczema Area and Severity Index (EASI) ≥21, and a lack of response, and intolerance or contraindication to treatment with cyclosporine. None of the patients included started treatment within a clinical trial. Patients were assessed by a clinician at baseline and 4 weeks after starting dupilumab.
The administration of dupilumab was started following the indications set forth in a data sheet. Patients who were on other systemic treatments for AD when they started dupilumab, continued to receive this treatment during the study follow-up, without any dosage changes. Topical emollients, corticosteroids or calcineurin inhibitors were maintained.
The statistical analysis was performed using IBM SPSS 22.0 (IBM SPSS Statistics Base) software, as shown in Table 1.
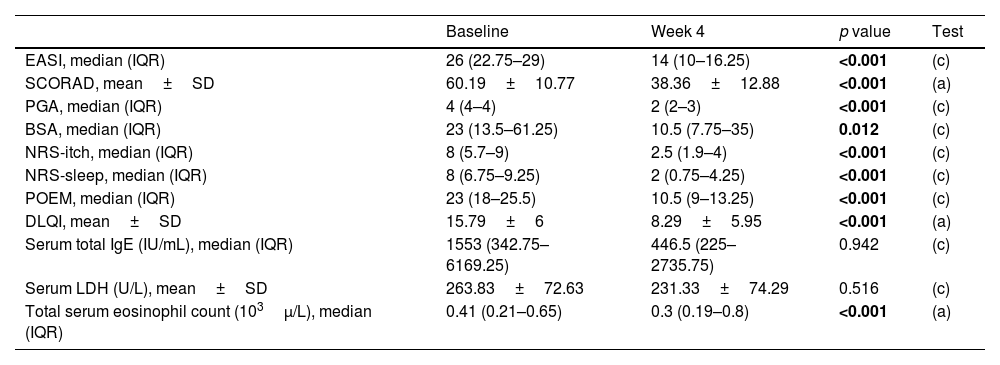
Comparison of clinical and analytical variables at baseline and after 4 weeks of treatment with dupilumab.
| Baseline | Week 4 | p value | Test | |
|---|---|---|---|---|
| EASI, median (IQR) | 26 (22.75–29) | 14 (10–16.25) | <0.001 | (c) |
| SCORAD, mean±SD | 60.19±10.77 | 38.36±12.88 | <0.001 | (a) |
| PGA, median (IQR) | 4 (4–4) | 2 (2–3) | <0.001 | (c) |
| BSA, median (IQR) | 23 (13.5–61.25) | 10.5 (7.75–35) | 0.012 | (c) |
| NRS-itch, median (IQR) | 8 (5.7–9) | 2.5 (1.9–4) | <0.001 | (c) |
| NRS-sleep, median (IQR) | 8 (6.75–9.25) | 2 (0.75–4.25) | <0.001 | (c) |
| POEM, median (IQR) | 23 (18–25.5) | 10.5 (9–13.25) | <0.001 | (c) |
| DLQI, mean±SD | 15.79±6 | 8.29±5.95 | <0.001 | (a) |
| Serum total IgE (IU/mL), median (IQR) | 1553 (342.75–6169.25) | 446.5 (225–2735.75) | 0.942 | (c) |
| Serum LDH (U/L), mean±SD | 263.83±72.63 | 231.33±74.29 | 0.516 | (c) |
| Total serum eosinophil count (103μ/L), median (IQR) | 0.41 (0.21–0.65) | 0.3 (0.19–0.8) | <0.001 | (a) |
IQR: interquartile range; SD: standard deviation; EASI: Eczema Area and Severity Index; SCORAD: scoring atopic dermatitis; PGA: physician global assesment; BSA: body surface area; NRS: numerical rating scale; POEM: patient-oriented eczema measure; DLQI: Dermatology Life Quality Index; IgE: immunoglobulin E; LDH: lactate dehydrogenase.
(a) Student's t-test assuming homogeneity of variances; (b) Student's t-test when homogeneity of variances is not met; (c) Mann–Whitney–Wilcoxon U test.
Bold values are the statistically significant variables (p < 0.05).
A total of 24 patients (17 men and 7 women) were included. All patients had severe AD at baseline, defined as an EASI ≥21, SCORAD ≥50, BSA ≥10, and PGA ≥3.
Other allergic disesases were reported by 17 patients (70.8%), with allergic rhinitis being the most frequent (70.8%), followed by allergic conjuntivitis (62.5%), bronchial asthma (45.8%), food allergies (29.2%) and nasal polyposis (8.3%).
The mean of previous systemic treatments was 3.54 drugs, with 19 patients (79.2%) undergoing three or more previous systemic treatments. All patients had used oral corticosteroids and cyclosporine in the past.
A total of 11 patients (45.8%) were undergoing systemic treatment for AD when they started dupilumab, 5 of whom (20.8%) were on corticosteroids, 5 (20.8%) on cyclosporine and 1 (4.1%) on azathioprine.
After 4 weeks on dupilumab, a statistically significant improvement was observed in all the measured variables as shown in Table 1.
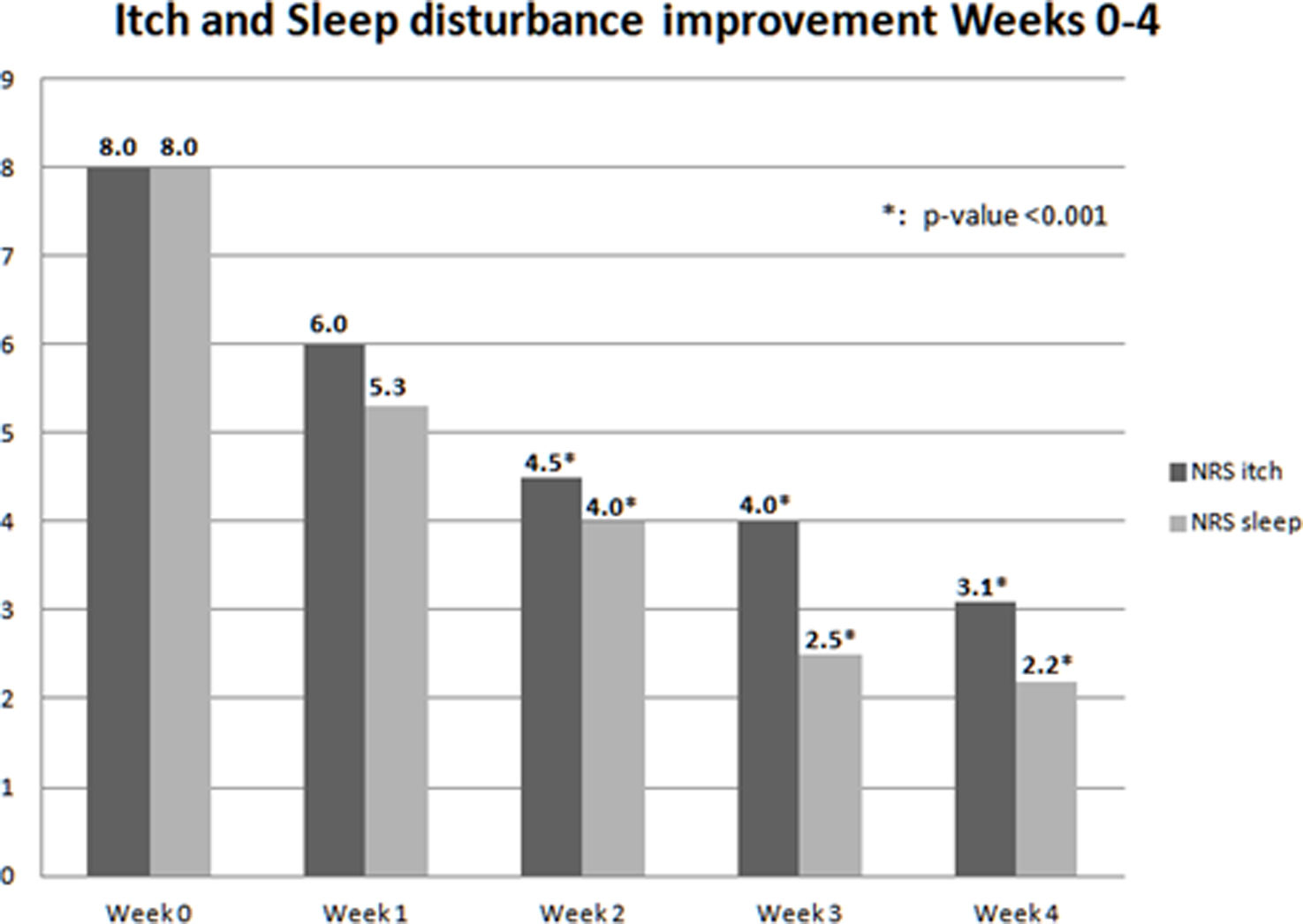
Variables NRS-itch and NRS-sleep were measured on a daily basis, obtaining a weekly mean shown in Fig. 1. An improvement in itching and sleep quality was observed since the first week of treatment, achieving statistical significance on weeks 2, 3 and 4.
These results are similar to those from other studies published in clinical trials and real-world studies.2,3 It remarkable that variables related to quality of life such as NRS-itch, NRS-sleep, or DLQI achieved a faster and greater improvement vs other variables measuring the severity of skin disease such as POEM, EASI or SCORAD, as seen in Fig. 1.
Given that a 4-week washout period of systemic immunosuppressive drugs went by in dupilumab pivotal clinical trials, it is unknown how to transition between classic systemic treatments for AD and dupilumab under real-world clinical practice conditions in which no washout periods are undertaken.
Following different author's proposals,4,5 in our sample, all patients on a classic immunosuppressant when dupilumab was started (11/24, 45.8%) went on with it without any dose changes within the first 4 weeks of treatment. None of them presented an initial exacerbation of AD. No negative impact in terms of safety was seen either.
Among the limitations of this study, those derived from the small sample size and short follow-up period are most notable. Studies conducted in the routine clinical practice with a larger number of patients and longer follow-up time will be necessary to evaluate the long-term effectiveness of dupilumab and establish differences between subgroups of patients. Among the study strengths, we can highlight its prospective design and the multiple scores and variables analyzed.
Conflict of interestsThe authors state that they have no conflict of interests.