Dupilumab is a new targeted therapy for severe atopic dermatitis (AD) with limited real-world evidence.
ObjectiveExplore our experience with dupilumab for AD in clinical practice at a tertiary care center.
Material and methodUnicentric observational retrospective study including adult and pediatric patients with severe AD receiving dupilumab between December 2017 and December 2021. The Eczema Area and Severity Index (EASI) score, Pruritus Numerical Rating Scale (P-NRS) and Sleep disturbance Numerical Rating Scale (S-NRS) were recovered to assess severity and response.
ResultsFifty-nine patients received dupilumab: 52, 48, 26 and 13 patients reached 6, 12, 24 and 36 months of treatment, respectively. The EASI-75 response rates were 94.2%, 95.8%, 92.3% and 100% at months 6, 12, 24 and 36. The EASI-90 response rates were 63.5%, 72.9%, 84.6% and 92.3% at months 6, 12, 24 and 36. The EASI <7 response rates were 92.3%, 91.7%, 88.5% and 100% at months 6, 12, 24 and 36. The P-NRS ≥4 reduction rates were 86%, 87.5%, 92.3% and 100% at months 6, 12, 24 and 36. The S-NRS ≥4 reduction rates were 82.7%, 85.4%, 100% and 100% at months 6, 12, 24 and 36. Adverse events were mild and occurred in 20.3% of patients, all of them adults.
ConclusionOur findings support dupilumab's favorable efficacy and tolerability profile in clinical practice. Dupilumab offers a rapid and sustained response, regardless of combined therapy. Longer follow-ups are still required to adequately assess its performance.
Dupilumab es una nueva terapia dirigida para la dermatitis atópica (DA) grave con una evidencia en la vida real aún limitada.
ObjetivoExplorar nuestra experiencia con dupilumab para la DA en práctica clínica en un centro terciario.
Material y métodoEstudio observacional retrospectivo y unicéntrico que incluye pacientes adultos y pediátricos con DA grave en tratamiento con dupilumab entre diciembre de 2017 y diciembre de 2021. La gravedad y la respuesta se evaluaron con las escalas Eczema Area and Severity Index (EASI), Pruritus Numerical Rating Scale y Sleep Disturbance Numerical Rating Scale.
ResultadosCincuenta y nueve pacientes recibieron dupilumab: 52, 48, 26 y 13 pacientes alcanzaron los 6, 12, 24 y 36 meses de tratamiento, respectivamente. La tasa de EASI-75 fue del 94,2; 95,8; 92,3 y 100% a los 6, 12, 24 y 36 meses, respectivamente. La tasa de EASI-90 fue del 63,5; 72,9; 84,6 y 92,3% a los 6, 12, 24 y 36 meses, respectivamente. La tasa de EASI <7 fue del 92,3; 91,7; 88,5 y 100% a los 6, 12, 24 y 36 meses, respectivamente. La Pruritus Numerical Rating Scale ≥4 fue del 86; 87,5; 92,3 y 100% a los 6, 12, 24 y 36 meses, respectivamente. La tasa de reducción Sleep Disturbance Numerical Rating Scale ≥4 fue del 82,7; 85,4; 100 y 100% a los 6, 12, 24 y 36 meses, respectivamente. Los eventos adversos fueron leves y ocurrieron en el 20,3% de los pacientes, todos adultos.
ConclusiónNuestros hallazgos apoyan el perfil favorable de eficacia y tolerabilidad de dupilumab en práctica clínica real. Dupilumab ofrece una respuesta rápida y mantenida, independientemente del uso de terapia combinada. Se requieren seguimientos más prolongados para evaluar su funcionamiento a largo plazo.
Atopic dermatitis (AD) is a chronic inflammatory skin disorder that is common among the adult population, with a general prevalence between 2.2% and 17.6% and with varying grades of severity.1 Eczematous lesions, xerosis and pruritus characterize this disease that typically begins during early childhood, although it may appear de novo in adults in up to 9–24.5% of cases.2 This condition may significantly impact patients’ quality of life, particularly in severe cases.3 The pathophysiology of AD involves a complex interaction of human and environmental factors and is centered around, but not limited to, an upregulated T helper 2 (Th2) pathway and increased levels of interleukins (IL)-4 and 13.4 Clinical guidelines have long included classic systemic immunosuppressants as treatment options for severe AD in both children and adults, when topical corticosteroids (TCS) and topical calcineurin inhibitors (TCI) are insufficient. The risks derived from broad immunosuppression and the need for monitorization during these treatments have motivated the search for both safer and more effective targeted therapies.5
Dupilumab is a monoclonal antibody that inhibits both IL-4 and IL-13 simultaneously through antagonism of IL-4 receptor a, which serves as a target for both of these interleukins implicated in the Th2 pathway.6 Clinical trials assessing this drug with and without background therapy with topical corticosteroids have found favorable results regarding its efficacy and safety for the treatment of moderate-to-severe AD.6–8 It is approved by the European Medicines Agency for its use in moderate-to-severe AD in patients aged 6 months or older, and is now included in the current European guidelines as an option for adults and children with severe AD.5 Although dupilumab appears to be an important addition to our therapeutic arsenal, data on its efficacy and safety in a real-world setting is still limited. The studies in clinical practice published so far report on adult patients followed for periods ranging mostly between 4 and 16 weeks, with few series reaching 52 or 84 weeks of follow-up.9–14 A multicenter series recently reported on a longer follow-up of 156 weeks.15
In this work, we report on our experience in clinical practice with dupilumab for the treatment of severe AD in adults and children at a tertiary referral center.
Materials and methodWe performed a unicentric observational retrospective study including all patients with severe AD treated with dupilumab at our tertiary referral center between December 2017 and December 2021. This study was approved by the Institutional Review Board. All patients included fulfilled diagnostic criteria for AD as per evaluation by dermatologists at our center.16 Patient data was recovered from electronic medical records and upon initiation of treatment. Patients were classified according to the time of disease onset (over or under 18 years of age) and baseline clinical phenotype, distinguishing between classic phenotype (predominantly flexural or head and neck dermatitis), erythroderma and exclusively prurigo-like lesions. Data on total serum immunoglobulin E (IgE) levels prior to treatment were recovered. The maximum value for IgE levels determined at our laboratory is 5000U/mL. Patients were also classified into allergic and non-allergic forms of AD according to their clinical parameters and total serum IgE levels.17,18 All erythrodermic patients were evaluated with full blood work-up and skin biopsy to rule out mycosis fungoides.
Upon initiation of dupilumab, patients already receiving systemic therapy maintained or discontinued the latter depending on our assessment of their individual risk of worsening. The duration of combined therapy was determined by the degree of control of their disease. Basic therapy and topical reactive and proactive strategies with TCS and TCI were maintained as recommended by the European guidelines.5 Standard treatment regimen with dupilumab was 600mg at first administration followed by 300mg every two weeks for patients weighing 40kg or above. For patients under 40kg, dupilumab was initiated with an induction phase of 300mg at weeks 0 and 2, followed by 300mg monthly. Exceptionally, in patients responding to therapy but whose improvement was partial or did not last until the following dose, therapy could be intensified by reducing the dosing interval to 300mg weekly.
The clinical parameters assessing AD severity were the Eczema Area and Severity Index (EASI) score, Pruritus Numerical Rating Scale (P-NRS) and Sleep disturbance Numerical Rating Scale (S-NRS). Therapeutic response was evaluated using the following variables: EASI score reduction of 50% (EASI-50), 75% (EASI-75) and 90% (EASI-90), significant reduction of P-NRS and S-NRS, defined as a reduction of ≥4 points, and achievement of a 0 or 1 on these two scales (P-NRS 0/1 and S-NRS 0/1). Information on therapeutic response and adverse events was recovered from follow-up visits around weeks 4, 12, and 24 and every 6 months thereafter. Regarding ocular symptoms, the term conjunctivitis was reserved for patients who developed ocular complaints plus conjunctival changes and who received this diagnosis after ophthalmological evaluation.
Data was processed using the Statistical Package for the Social Sciences software version 25 and information was portrayed using figures generated with Microsoft Excel version 16.35. Descriptive statistics were performed using the mean and range to express quantitative variables, and absolute frequencies and percentages to describe qualitative variables. Statistical analysis was performed to compare the therapeutic response between patients initiating dupilumab with and without combined therapy, setting significance at p<0.05. Qualitative variables were compared using the chi-squared test and quantitative variables were compared using the t-Student test or the U Mann–Whitney test for variables following a normal or non-normal distribution, respectively.
ResultsA total of 59 patients began therapy with dupilumab for moderate-to-severe AD during the study period. Demographic characteristics are shown in Table 1. There were four patients (6.8%) under the age of 12 who were receiving dupilumab off-label. Details on patients’ AD history are shown in Table 2. Of the 21 patients (35.6%) with adult-onset disease, one developed AD during elderhood. There were no differences in baseline characteristics between patients with onset under or over 18 years of age and between allergic and non-allergic forms of AD.
Demographic characteristics of the patients (n=59).
| Variable | Value |
|---|---|
| Age, years, mean [range] | 34.6 [5–91] |
| Under 18 years, n (%) | 9 (15.3) |
| Under 12 years, n (%) | 4 (6.8) |
| Male, n (%) | 36 (61) |
| BMI, kg/m2, mean [range] | 23.6 [11–33] |
| Underweight, n (%) | 4 (6.8) |
| Normal weight, n (%) | 34 (57.6) |
| Overweight, n (%) | 16 (27.1) |
| Obesity, n (%) | 5 (8.5) |
| CVRF, n (%) | 15 (25.4) |
| Smoking, n (%) | 4 (6.8) |
| Dyslipidemia, n (%) | 4 (6.8) |
| Arterial hypertension, n (%) | 3 (5.1) |
| Diabetes mellitus, n (%) | 2 (3.4) |
| Allergic comorbidities, n (%) | 36 (61) |
| Rhinitis, n (%) | 29 (49.2) |
| Asthma, n (%) | 25 (42.4) |
| Food allergy, n (%) | 8 (13.6) |
| Psychiatric disease, n (%) | 3 (5.1) |
| Depression, n (%) | 1 (1.7) |
| Anxiety, n (%) | 1 (1.7) |
| Schizophrenia, n (%) | 1 (1.7) |
BMI, body mass index; CVRF, cardiovascular risk factors.
Characteristics of patients’ history of atopic dermatitis (n=59).
| Variable | Value |
|---|---|
| Age of onset, years, mean [range] | 15.2 [1–88] |
| Under 18 years, n (%) | 38 (64.4) |
| Over 18 years, n (%) | 21 (35.6) |
| Clinical phenotype | |
| Classic, n (%) | 49 (83.1) |
| Erythroderma, n (%) | 7 (11.9) |
| Prurigo, n (%) | 3 (5.1) |
| Baseline IgE levels, U/mL, mean [range] | 2703.6 [13–5000*] |
| Form of atopic dermatitis | |
| Allergic | 53 (89.8) |
| Non-allergic | 6 (10.2) |
| Baseline EASI, mean [range] | 29.6 [12–58] |
| Baseline P-NRS, mean [range] | 8.4 [4–10] |
| Baseline S-NRS, mean [range] | 7.1 [0–10] |
| History of hospitalization, n (%) | 10 (16.9) |
| Previous systemic therapy | |
| No. of therapies, mean [range] | 3.2 [1–8] |
| 1 or 2 therapies, n (%) | 26 (44.1) |
| 3 or more therapies, n (%) | 33 (55.9) |
| Corticosteroids, n (%) | 59 (100) |
| Cyclosporine A, n (%) | 49 (83.1) |
| Azathioprine, n (%) | 25 (42.4) |
| Methotrexate, n (%) | 19 (32.2) |
| Phototherapy, n (%) | 14 (23.7) |
| Mycophenolate mofetil, n (%) | 3 (5.1) |
| Omalizumab, n (%) | 5 (8.5) |
| IL-12/23 inhibitor, n (%) | 4 (6.8) |
| IL-17C inhibitor – clinical trial, n (%) | 3 (5.1) |
| IL-13 inhibitor, n (%) | 3 (5.1) |
| JAK inhibitor, n (%) | 2 (3.4) |
| Combined systemic therapy, n (%) | 25 (42.4) |
| Cyclosporine A, n (%) | 13 (22) |
| Corticosteroids, n (%) | 9 (15.3) |
| Methotrexate, n (%) | 3 (5.1) |
| Azathioprine, n (%) | 1 (1.7) |
IgE, immunoglobulin E; EASI, Eczema Area and Severity Index; P-NRS, Pruritus Numerical Rating Scale; S-NRS, Sleep disturbance Numerical Rating Scale; IL, interleukin; JAK, Janus kinase.
Dupilumab was started in combination with a classic systemic drug in 25 cases (42.4%). The concomitant drug was administered for a mean duration of 3 months (range 1–18) before continuing with dupilumab as monotherapy. Twenty patients (33.9%) received the combined therapy for ≤3 months, three patients (5.1%) for 3–6 months and two patients for >6 months (8 and 18 months). Details on the concomitant drugs are shown in Table 2. Dupilumab was administered following the standard regimen in 57 cases (96.6%) and the remaining 2 (3.4%) were intensified to 300mg weekly; 1 due to partial response and 1 for progressive loss of improvement after a complete response. The mean follow-up was 23 months (range 3–48), with 52 patients (88.1%) reaching the 6-month time point, 48 patients (81.4%) reaching the 12-month time point and 26 patients (44.1%) reaching the 24-month time point. A total of 13 patients (22%) reached a follow-up of 36 months.
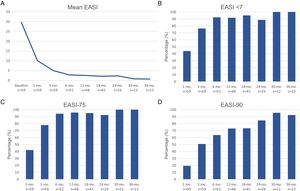
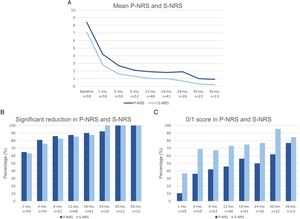
Therapeutic response in terms of mean EASI as well as EASI <7, EASI-75 and EASI-90 response rates is illustrated in Fig. 1. The subjective response in terms of P-NRS and S-NRS is illustrated in Fig. 2, including mean values, significant reduction rates and 0/1 response rates. Exact values of therapeutic response in terms of EASI, P-NRS and S-NRS are shown in Table 3.
Therapeutic response to dupilumab in terms of the subjective parameters Pruritus Numerical Rating Scale (P-NRS) and Sleep Numerical Rating Scale (S-NRS) from baseline through month 36. (A) Evolution of mean P-NRS and S-NRS. (B) P-NRS and S-NRS significant reduction rates. (C) P-NRS 0/1 and S-NRS 0/1 response rates.
Therapeutic response to dupilumab for severe atopic dermatitis in terms of Eczema Area and Severity Index (EASI), Pruritus Numerical Rating Scale (P-NRS) and Sleep Numerical Rating Scale (S-NRS), from baseline through month 36.
| Response parameter | Follow-up (months) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline(n=59) | 1(n=59) | 3(n=59) | 6(n=52) | 12(n=48) | 18(n=41) | 24(n=26) | 30(n=22) | 36(n=13) | |
| EASI | |||||||||
| Mean EASI | 29.6 | 10.1 | 5 | 2.8 | 2.5 | 2.1 | 2.3 | 0.8 | 0.7 |
| EASI <7 | – | 43.9 | 76.3 | 92.3 | 91.7 | 95.1 | 88.5 | 100 | 100 |
| EASI-50 (%) | – | 82.5 | 96.6 | 98.1 | 100 | 100 | 100 | 100 | 100 |
| EASI-75 (%) | – | 42.1 | 78 | 94.2 | 95.8 | 95.1 | 92.3 | 100 | 100 |
| EASI-90 (%) | – | 19.3 | 50.8 | 63.5 | 72.9 | 73.2 | 84.6 | 95.5 | 92.3 |
| P-NRS | |||||||||
| Mean P-NRS | 8.4 | 4.2 | 2.7 | 2.1 | 1.9 | 1.8 | 1.9 | 1 | 0.9 |
| Significant reduction of P-NRS (%) | – | 64.9 | 81 | 86 | 87.5 | 90.2 | 92.3 | 100 | 100 |
| P-NRS 0/1 (%) | – | 10.5 | 36.2 | 42 | 45.8 | 56.1 | 50 | 61.9 | 76.9 |
| S-NRS | |||||||||
| Mean S-NRS | 7.1 | 2.8 | 1.6 | 1.3 | 1 | 1 | 0.7 | 0.3 | 0.2 |
| Significant reduction of S-NRS (%) | – | 63.2 | 75.9 | 82.7 | 85.4 | 87.5 | 100 | 100 | 100 |
| S-NRS 0/1 (%) | – | 36.8 | 69 | 67.3 | 72.9 | 75 | 76.9 | 95.5 | 84.6 |
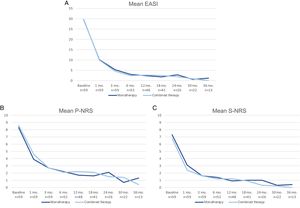
A comparison of the change in mean EASI, P-NRS and S-NRS between patients initiating dupilumab as monotherapy or in combination with another systemic drug is shown in Fig. 3. Statistical analysis found no significant differences in mean EASI, P-NRS and S-NRS values at baseline or at any of the time points. Further analysis found no statistically significant differences in EASI <7, EASI-50, EASI-75 or EASI-90 response rates, significant reduction in P-NRS and S-NRS, and achievement of P-NRS 0/1 and S-NRS 0/1, at any of the time points. We also did not find statistically significant differences in response parameters between patients with onset under or over 18 years of age, between clinical phenotypes and between allergic and non-allergic forms of AD.
Comparison between patients initiating dupilumab as monotherapy and in combination with another systemic drug in terms of mean Eczema Area and Severity Index (EASI), Pruritus Numerical Rating Scale (P-NRS) and Sleep Numerical Rating Scale (S-NRS), from baseline through month 36. (A) Evolution of mean EASI. (B) Evolution of mean P-NRS. (C) Evolution of mean S-NRS.
Regarding drug safety, we identified adverse events in 12 patients (20.3%). Eight patients (13.6%) presented ocular symptoms; four patients (6.8%) developed conjunctivitis and four (6.8%) complained of ocular pruritus without conjunctivitis. Four of these seven patients had a history of allergic rhinoconjunctivitis. Ocular symptoms were successfully managed with topical treatments and artificial tears. We identified one case (1.7%) of each of the following adverse events: psoriasis, head and neck erythema, reaction at injection site, herpes simplex infection, dyspepsia, headache and asthenia. None of the adverse events were severe and they did not motivate discontinuation of dupilumab. All the adverse events identified occurred in adult patients.
Dupilumab had to be discontinued in a total of seven patients (15.3%). Reasons for discontinuation were gestation in two patients (3.4%), loss of response in one patient (1.7%) and insufficient response in six patients (10.2%), including one of the patients receiving intensified therapy. Six of the seven patients presenting treatment failure had previously received only classic systemic therapies (corticosteroids and cyclosporine A). One patient had previously failed to respond to omalizumab and none of them had previously received with JAK inhibitors. Treatment was suspended after a mean duration of 14.1 months (range 4–24). No patient had to abandon therapy due to tolerability issues.
DiscussionUntil the recent introduction of dupilumab in our therapeutic arsenal against AD, this condition has long been in need for disease-specific treatments offering high efficacy rates and improved safety and tolerability profiles. Dupilumab appears to meet this need according to the results reported in clinical trials.6–8 However, conditions in clinical trials often differ from real-world settings, which prompts the need for evaluating its use in clinical practice. We report on our real-world experience with adults and children receiving dupilumab for severe AD. The demographic and disease characteristics in our series are similar to that of the largest clinical practice series published to date.19 The mean EASI scores were 29.3 and 28, respectively, which are also comparable to those reported in clinical trials.6–8 These values are indicative of the severity of these patients’ AD which, together with the proportion of patients failing to previous systemic therapies, reflect on the profile of patient treated with dupilumab in real-world settings.12,19,20
The evolution in mean EASI, P-NRS and S-NRS scores and the different response rates observed in our series indicate that patients generally experienced a faster response to dupilumab than that described in clinical trials.8 A significant improvement was seen early on, with over 80% of patients achieving an EASI-50 reduction already after 1 month and over 90% after 3 months. The EASI-75 and EASI-90 response rates, despite being more demanding outcomes, were still reflective of the rapidity and magnitude of this improvement; up to 75% and 50% after 3 months, respectively. These rates were similar to, but slightly above, the pooled outcomes for the 1- and 3-month time points reported in a recent meta-analysis.9 This fast improvement was also reflected by the P-NRS and S-NRS response rates as subjective outcomes, particularly in regards to pruritus. Over 60% and 80% of patients experienced a significant reduction in P-NRS after only 1 and 3 months of treatment, respectively.
Our follow-up data from month 6 through month 36 indicates there is a sustained response to dupilumab after the initial improvement, as the EASI-50 reduction was maintained by all patients continuing therapy for 6 months or longer. Furthermore, we observed a gradual decrease in mean EASI, P-NRS and S-NRS scores and a progressive increase in EASI-75, EASI-90 and the P-NRS and S-NRS significant reduction rates over time. This reflects on a further improvement with longer treatment durations. The largest series with prolonged follow-up reporting on these parameters also described very favorable response rates in terms of EASI-50 (97.2%), EASI-75 (73.3%), EASI-90 (37.5%) and P-NRS improvement of ≥4 points (82.6%) after 52 weeks.12 Similar rates were found in other large series from national registries reaching 52 and 84 weeks of follow-up.13,14 The multicenter series with 156 weeks of follow-up reported on high response rates in terms of SCORAD (SCORing Atopic Dermatitis) and pruritus VAS (Visual Analogue Scale) reduction (88.2% and 77.1%, respectively).15 It is worth noting the effect of dupilumab on the pruritus, as the P-NRS significant reduction rates in our series are consistently above 85% from month 6 through month 36. Furthermore, we observed that almost 50% of patients reaching 24 months of treatment presented a P-NRS score of 0/1, that is, a state of none or minimal pruritus. This is, in the authors’ opinion, particularly significant given the importance of pruritus in the course of AD and how difficult it often is to control. The erythrodermic phenotype of AD is an interesting form of this disease for which there is still little evidence regarding response to treatments. All seven erythrodermic patients in this study achieved an EASI-90 response after a mean of 7 months and seven patients achieved P-NRS and S-NRS scores of 0/1 after a mean of 14 and 8 months, respectively. Their favorable objective and subjective response did not differ from that of the classical or prurigo phenotypes.
The response over time of patients initiating dupilumab alone was similar to that of patients receiving combined therapy, as observed by the data shown in Fig. 3. Provided that the concomitant systemic drug was administered only during the first 1–3 months in the majority of patients, it is of interest that the decrease in mean EASI, P-NRS and S-NRS was comparable during this period. Given that the statistical analysis found no significant differences in the mean parameters and response rates studied, it seems that patients may experience a fast improvement upon initiation of dupilumab regardless of whether they receive combined therapy. We believe the decision of initiating dupilumab in combination with another systemic drug must not be motivated by the need for a faster response, and should rather depend solely on the previous degree of control or stability of the disease.
The safety and tolerability profile of dupilumab was favorable, as our observed rate of adverse events was 20.3% and no severe adverse events were identified. This is in line with that reported in the largest series to date, where data on 534 patients also support dupilumab's good safety profile. Nevertheless, the rate of adverse events varies significantly, up to 71%, among the different real-world series in the literature.9,21 It is worth highlighting that none of the adverse events occurred among the patients under 18 years or among the off-label uses below the age of 12, supporting the findings so far on its safety and tolerability in children.22–24
Special awareness has been raised regarding ocular adverse events, conjunctivitis in particular, given their reported rates in clinical trials.6–8 The incidence of conjunctivitis in clinical practice ranges from as low as 6% up to 70%, depending on the study.9,25 Although generally mild, severe forms may lead to discontinuation of dupilumab.26,27 In our experience, only four patients (6.8%) developed true conjunctivitis. These patients presented mild forms that resolved with topical treatment. The variability in the incidence of conjunctivitis could be due, among other factors, to different diagnostic criteria and the involvement of an ophthalmologist in the diagnosis.28,29 Nevertheless, its causal, rather than temporal, relationship is yet to be established and the possible mechanisms and predictors of its development are unclear.19,30 We encountered few non-ocular adverse events, all of which have already been described in the literature.9,22
Regarding discontinuation of dupilumab due to treatment failure, it is worth noting that we could not associate this lack of response to previous failure to other biologics or JAK inhibitors. This is perhaps due to the availability of dupilumab before the introduction of the latter in our therapeutic arsenal.
Our findings should be interpreted taking into consideration this study's limitations, including its observational and retrospective nature, short follow-up in a subset of patients, limited sample size and lack of control patients.
In conclusion, this work supports dupilumab's favorable efficacy and safety profile for the treatment of severe AD in clinical practice, both in adults and children. Dupilumab offers a rapid improvement in clinical lesions and pruritus, with or without concomitant therapy. This response is sustained and may be followed by further improvement over time. Adverse events are generally mild, making dupilumab a tolerable and convenient option for patients that would otherwise require broad immunosuppressants. Longer follow-ups are still required to adequately assess its performance and to develop future strategies in the long-term management of AD with dupilumab.
Conflict of interestsThe authors declare that they have no conflict of interest.