The incidence of herpes zoster (HZ) is 42 cases per 100 000 people per year. Infection in children is very rare. The varicella vaccine offers 90% protection against chickenpox and nearly 100% protection against severe cases.1 Immunization against varicella was initially expected to cause a decrease in the incidence of HZ. However, the vaccine virus can cause a latent infection that later manifests as HZ. Recent studies have also demonstrated that the risk of HZ in children who have had natural varicella infection is significantly higher than in vaccinated children with no history of chickenpox.2,3
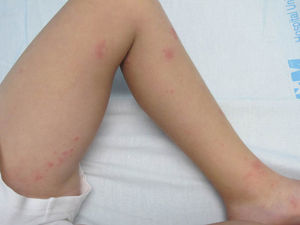
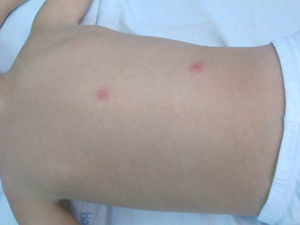
Case DescriptionA 6-year-old boy came to our hospital for assessment of pruritic lesions and pain predominantly in the lower left limb. The patient had received a single dose of the varicella-zoster virus (VZV) vaccine at the age of 15 months. Physical examination revealed vesicles grouped on an erythematous base. The lesions followed a metameric distribution on the medial aspect of the left thigh (Fig. 1). Other scattered lesions were observed on the contralateral lower limb and the lumbar region (Fig. 2). Complete blood count and biochemistry profile were normal except for mild lymphopenia (1.91×103/μL [normal values: 2.70-12.60]). VZV serology was positive for immunoglobulin G anti-VZV antibody and polymerase chain reaction of a smear from one of the vesicles was positive. After treatment with oral aciclovir at a dose of 250mg/m2 every 8hours and follow-up after 4 days, clear improvement was observed in the lesions and the pain, with the alteration having disappeared in the course of treatment.
DiscussionSince 1998, there have been several reports of HZ in children after vaccination. Molecular biology techniques have been used to differentiate between wild-type VZV and the vaccine strain of the virus (Oka).
The estimated incidence of HZ in vaccinated children varies widely between studies, between 262.1 cases per 100 000 person-years by natural infection and 93.3 cases per 100 000 person-years after vaccination.2,4 This incidence may be underestimated due to underdiagnosis associated with unfamiliarity with HZ or with the low incidence of the entity in children and the atypical nature of postvaccination cases. Cases of HZ caused by the vaccine strain of the virus exhibit clinical differences with respect to cases caused by wild-type VZV; this could lead to a lower consultation rate in the first group. Among other differences, postvaccination HZ lesions tend to be smaller and less painful, with fewer vesicles, and the lesions predominantly appear on the lumbosacral dermatomes.5 In our case, however, the form of presentation was considerably different and atypical, as the patient was a child with disseminated lesions and intense pain in the affected metamere.
Given the greater susceptibility of children with leukemia to developing HZ, studies have been carried out in this population to assess the incidence of this complication. However, it is important to note that routine VZV vaccination is not recommended in immunocompromised patients because the vaccine contains a live attenuated virus. In children with leukemia, the incidence of HZ was 3 times higher in those who had had natural varicella infection than in those who had received the vaccine.6
Age at vaccination could be an important factor influencing the appearance of postvaccination HZ because immunogenicity is lower at younger ages. Reactivation of the Oka virus is more frequent in vaccinated children with low anti-VZV titers.7 These data are consistent with evidence that suggests that varicella infection in the first year of life increases considerably the risk of HZ during childhood. This might be due to the fact that immunity levels are lower in children who contract varicella at a younger age.8 The time to manifestation of HZ after contact with the virus is also related to the age at which contact occurs. The mean duration in children between exposure to varicella and development of HZ has been estimated at 4.12 years, and significantly shorter in children diagnosed with varicella before age 2 years.2
One factor that appears to increase the risk of HZ after vaccination against varicella is the appearance of exanthema after administration of the vaccine. The relative risk of HZ in vaccinated children has been found to be 5.75 times greater in children who develop a vaccine-associated skin rash. According to one hypothesis, the skin lesions could allow the virus to enter the cutaneous nerves and establish a latent infection. The lower incidence of HZ after vaccination could be due to the fact that the attenuated varicella vaccine is less able to access the sensory nerves.9
In conclusion, we present a case of disseminated HZ in a boy with a history of incomplete vaccination against VZV. Although this complication is described in the literature, no increase in incidence after vaccination has been observed. Incomplete vaccination probably led to a waning of immunity that favored the development of HZ.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Rueda JM, Feito-Rodríguez M, Nieto D, de Lucas-Laguna R. Herpes zóster diseminado posvacunal en un niño sano. Actas Dermosifiliogr. 2017;108:587–588.