Smartphone applications (apps) are of particular interest to dermatologists, and some 229 already exist in the categories of general dermatology, self-surveillance/diagnosis, disease guides, patient education, sun protection, calculators, teledermatology, dermoscopy, etc.1–3 All of these apps are potentially very useful, although the use that dermatologists and patients make of them has not yet been studied.
The design and preparation of personalized medication solutions or compounds requires training and practice on the part of both the dermatologist and the pharmacist. The dermatologist must specify the quantity and quality of the active ingredients and the most appropriate excipients depending on the characteristics of the patient's skin disease. The pharmacist must prepare the medication using the best available technique.4
To aid clinicians in this task, we have developed Dermacomp, an iPhone application that helps the dermatologist to create a customized medication using a quick, easy, and intuitive process that facilitates the design of the formula. Dermacomp also does the following: 1) improves the exchange of information between the dermatologist and the compounding pharmacist, including a large amount of information not normally found in traditional compounding prescriptions (such as, for example, patient characteristics, site of application, and vehicle type); 2) achieves better therapeutic results because it adapts the drug to the specific needs of each patient; and 3) displays the most appropriate active ingredients and vehicles for each disease and site, increasing the safety of dosing ranges and optimizing consultation time.5
Dermacomp was designed to run on devices using the iOS operating system (version 7 or higher) and is optimized for iPhone 4, 5, and 6. The scientific content was created by a dermatologist and a compounding pharmacist, and the app was developed using the Objective-C object-oriented language and Apple Xcode development tools. Management and storage of data is handled with Apple Frameworks and version control with the GIT system. Finally, the app was validated on several Apple devices.6
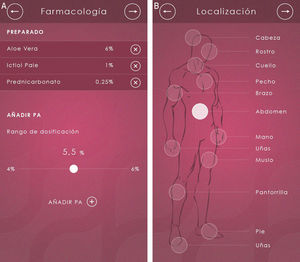
When the dermatologist opens Dermacomp, the app displays a list of the 5 skin disorders for which compounded medications are most often used: psoriasis, alopecia, oral and genital mucosal disorders, rosacea, and seborrheic dermatitis (Fig. 1A). Once the appropriate disorder has been selected, the user only has to complete 6 rapid and intuitive steps:
Step 1. Introduce patient characteristics, including age, drug allergies or adverse reactions and, in the case of a woman, pregnancy status, Based on this information, the app will exclude any active ingredients or vehicles unsuitable for the patient and adjust maximum concentration levels, especially in the case of children and pregnant women.
Step 2. Mark the site of the lesion(s) on a graphic representation of a human figure (Fig. 1B).
Step 3. Depending on the diagnosis selected, the app asks the dermatologist to specify either the type of skin disease to be treated (in the case of alopecias or mucosal disorders, for example) or the characteristics of the lesion (in the case of a psoriatic lesion, for example, which can be more or less hyperkeratotic).
Step 4. In this step the dermatologist has 2 options:
- 1.
To choose from 1 or 2 predefined formulas designed for each disorder, which take into account the site and lesion type. This is a good option for specialists who do not have enough experience in the design and prescription of customized medications. These preset options do not, however, take into account the patient's age, intolerance, or pregnancy status.
- 2.
To design a suitable medication using the app's decision-making algorithm. In this case, the app displays the active ingredients that can be used in the formula depending on the disease specified (except those excluded in step 1 by the age and intolerance criteria or the patient's pregnancy status). Possible incompatibilities between active ingredients were also taken into account and exhaustively assessed in the design of this app. The app can accept up to 6 active ingredients. For each ingredient the dermatologist must choose the desired concentration within a predefined range.
Step 5. Specify frequency of application, duration of treatment, and the percentage of body surface area affected.
Step 6. Vehicle selection, which is determined by the criteria established by the prescriber in the earlier steps. In this way, the app greatly reduces the range of possibilities for the dermatologist so that the decision is guided to a large extent by the ideal choice of vehicle.
The application then proposes 2 formulas and the possible vehicles, which are compatible with the criteria entered throughout the 6 previous steps.
The app was tested in a pilot study under conditions of normal use in clinical practice by 8 dermatologists with training and experience in compounding.7 After using the app, each dermatologist assessed it using a specifically designed questionnaire made up of 4 blocks of questions on the following topics: content, navigation, design, and usefulness. All the respondents reported that the content and services offered by Dermacomp were useful in their everyday clinical practice, and 72% thought that the app could improve their ability to prescribe personalized medications. Moreover, 43% considered that the final formulation proposed by the app was complete and included all the necessary information; in response to a question asking for possible improvements they said that the app should also provide price and dosage information (Table 1).
Main Results From the Assessment Questionnaire.
| Block | Results |
|---|---|
| Content | - The graphic representations of the different sites are sufficiently clear and descriptive. (100%) - The app includes all the pharmacological groups and active ingredients needed to ensure correct prescription. (86%) - Handling of treatment regimens is clear and simple. (57%) - The way the vehicle is selected works well. (100%) - The final formulation suggested by the app is complete and includes all the necessary information. (43%) |
| Navigation | - Navigation from screen to screen is simple and intuitive (100%) |
| Design | - The app is well designed. (100%) - The order of the contents is appropriate. (100%) |
| Usefulness | - The content and services provided by this app are useful in my daily clinical practice. (100%) - This application could improve the prescription of customized medications. (72%) - This tool is much more useful or considerably more useful than the other tools designed to aid the prescription of compounded medications. (100%) |
Dermatologists can consult all the features of the app and download it to a mobile from the website www.dermacomp.com.
FundingThe Dermacomp app was financed by the Instituto Aragonés de Ciencias de la Salud (IACS), a body that manages research activities within the health service of the Autonomous Community of Aragon. However, in terms of content the app is totally independent and the IACS did not intervene in any way in the process of its development.
Please cite this article as: Abarca Lachén E, Gilaberte Y. Dermacomp: aplicación para iPhone® para el diseño de medicamentos tópicos individualizados dirigido a dermatólogos. Actas Dermosifiliogr. 2016;107:622–624.