Glomuvenous malformations are hamartomatous lesions characterized by the presence of glomus cells in the vascular smooth muscle. We present the clinical and color Doppler ultrasound features of a series of 13 cases of histologically confirmed glomuvenous malformations. In all cases, the ultrasound study revealed moderately delimited superficial dermal and hypodermal pseudonodular structures of mixed echogenicity, with hypoechoic and heterogeneous areas and anechoic, pseudocystic tubular and lacunar zones. Arterial and venous vessels, mainly with a low flow (≤ 15cm/s) were observed in 85% of patients, but no arteriovenous shunts were present. Deeper structures were not affected and no phleboliths were detected. The clinical and ultrasound findings could facilitate diagnosis, surgical planning, and noninvasive follow-up in these tumors.
Las malformaciones glomuvenosas son hamartomas originados por la presencia de células glómicas en el músculo liso de las estructuras vasculares. Presentamos una serie de 13 casos de malformaciones glomuvenosas evaluadas clínicamente, estudiadas con ecotomografía Doppler color y confirmadas histológicamente. En las ecografías se observaron en todos los casos formaciones dérmicas e hipodérmicas superficiales, moderadamente delimitadas, de ecoestructura mixta, pseudonodulares, hipoecogénicas y heterogéneas con áreas tubulares y lacunares anecogénicas pseudoquísticas. El 85% de los casos demostró presencia de vasos arteriales y venosos, con predominio de los de baja velocidad (≤ 15cm/s) sin shunts arteriovenosos. No se visualizó compromiso de estructuras profundas ni flebolitos.
Los hallazgos clínicos y ecográficos podrían ayudar a precisar mejor el diagnóstico, la planificación quirúrgica o el seguimiento no invasivo en estas entidades.
Glomuvenous malformations (GVMs) are hamartomatous lesions characterized by the presence of glomus cells in the vascular smooth muscle.1 Unlike most vascular malformations, histologic examination of GVMs reveals glomus cells. Unlike glomus tumors, histology does not show a hyperplastic or proliferative tumoral pattern of the glomus cells.1–4 The glomus cells correspond to modified smooth muscle cells surrounding arteriovenous anastomoses in the neuromyoarterial plexuses, which regulate blood flow and temperature.1,5
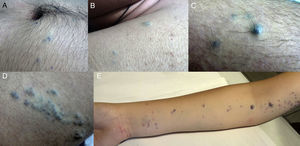
In more than 60% of cases, GVMs follow an autosomal dominant inheritance pattern. Diagnosis is based on clinical suspicion in the presence of blue or violaceous nodular or elongated structures that are sensitive to the touch, although to a lesser degree than glomus tumors. GVMs are often arranged linearly and sometimes have a dermatomal distribution.1,2
In recent years, a growing number of studies have demonstrated the utility of color Doppler ultrasound in the diagnosis of skin diseases, including vascular malformations and glomus tumors.6,7
We present the clinical and color Doppler ultrasound features of a series of 13 cases of histologically confirmed glomuvenous malformations (Table 1). All Doppler ultrasound studies were ordered by a physician after a dermatologic evaluation and carried out in accordance with the medical ethics principles of the Declaration of Helsinki.
Clinical and Sonographic Characteristics.
| Case | Sex | Age, y | No. of Segments | Description | Involvement | Flow | Flow Rate, cm/s |
|---|---|---|---|---|---|---|---|
| 1 | M | 9 | 2 | Hand and foot | Multiple | Arterial and venous | 5.7 |
| 2 | F | 14 | 2 | Thigh and leg | Multiple | None | 0 |
| 3 | M | 66 | 1 | Lower back | Single | Arterial and venous | 19.7 |
| 4 | F | 43 | 1 | Heel | Single | Arterial | 5.4 |
| 5 | F | 8 | 5 | Arm, forearm, costal region, dorsal region, knee | Multiple | Arterial and venous | 5.5 |
| 6 | F | 9 | 1 | Foot | Single | Arterial and venous | 4.4 |
| 7 | M | 15 | 4 | Periumbilical area, arm, forearms | Multiple | Arterial and venous | 3.3 |
| 8 | M | 14 | 1 | Chest | Multiple | Arterial and venous | 9.1 |
| 9 | F | 16 | 1 | Thigh | Single | Arterial and venous | 4.4 |
| 10 | F | 43 | 1 | Anterior abdominal wall | Multiple | Arterial and venous | 6.7 |
| 11 | M | 14 | 2 | Arm, thigh | Multiple | Arterial and venous | 4.2 |
| 12 | F | 34 | 1 | Forearm | Single | Arterial and venous | 3.8 |
| 13 | F | 30 | 2 | Dorsal region, lower back | Multiple | Arterial and venous | 19.4 |
Abbreviations: F, female; M, male.
Thirteen patients were evaluated (62% men and 38% women; mean [SD] age, 15 [18] years; range, 8-66 years). Bluish-violaceous nodules sensitive to the touch were present in all patients. Multiple segments (2 or more) were affected in 62% of patients and only 1 site was affected in 38% of patients. The number of body segments affected in each patient ranged from 1 to 5, with a mean of 2 segments affected per patient (Table 1) (Fig. 1).
Ultrasound ExaminationAll patients were evaluated with color Doppler ultrasound with a compact high-frequency linear transducer (LOGIQ E9 XDclear, General Electric; maximum frequency 18MHz).
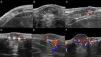
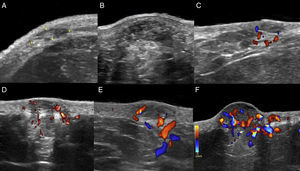
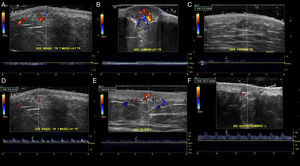
The location (dermis or hypodermis), nature (solid, cystic, mixed), borders, echogenicity (hyperechoic, hypoechoic, anechoic), presence, type (arterial, venous, both), and flow velocity (cm/s) were described (Table 1). In all cases, color Doppler ultrasound study revealed moderately delimited superficial dermal and hypodermal pseudonodular structures of mixed echogenicity, with hypoechoic and heterogeneous areas and anechoic, pseudocystic tubular and lacunar zones. Arterial and venous vessels, mainly with low-flow (≤ 15cm/s), were observed in 85% of patients, but no arteriovenous shunts were present. Deeper structures were not affected and no phleboliths were detected (Figs. 2 and 3).
In all cases, histologic examination revealed lesions composed of congestive blood vessels lined by a typical thin endothelium and surrounded by cells with a glomus appearance. Immunohistochemistry was positive for actin and negative for desmin.
DiscussionClinical diagnosis of GVM can be challenging. The use of ultrasound can help to clarify the diagnosis. More than 80% of patients with glomus tumors initially receive an incorrect clinical diagnosis, with a mean duration of 14 years from onset of symptoms to correct diagnosis.8
Although GVM could be considered a variant of glomus tumor or venous vascular malformation, the combination of clinical, sonographic, and histologic data and the presence of multiple, congenital, linearly distributed, sensitive but not highly painful lesions suggests a different entity.
In an ultrasound study, GVM is differentiated from glomus tumors by the absence of well-defined hypoechoic nodular formations, which are characteristic of intra- and extradigital glomus tumors.6,9
Sonographic differential diagnosis with arteriovenous vascular malformations is possible because spectral analysis in GVM usually does not show arterialized venous flow-through suggestive of arteriovenous communications or flow velocities>15cm/s.
GVM is distinguished from venous vascular malformations by the presence of low-flow arterial vessels with concomitant venous vascular structures.
The frequent finding of low arterial and venous flow in GVM suggests that glomuvenous is not the most accurate term, as it does not reflect the arterial component of this entity. A less confusing and more anatomically correct name would be glomus vascular malformation.
The utility of ultrasound in the study of GVM is limited by the sensitivity of the ultrasound equipment currently available, which is capable of detecting flow velocities of ≥ 2cm/s. Therefore, vessels with capillary, slow, or ectatic flow would not be perceptible in color Doppler spectral analysis.
Moreover, color Doppler ultrasound can help to rule out subclinical foci of lesions and areas of intralesional thrombosis and to detect and define the lesion's precise location, extent, and relationship with adjacent structures, which can be helpful if surgical intervention is necessary.6,7
However, the use of color Doppler ultrasound in dermatology requires training in the operation of the equipment, a constant practice, and the use of a standardized protocol.10
In conclusion, clinical-sonographic correlation could help to improve diagnostic accuracy in GVM and assist in surgical planning or noninvasive follow-up.
Ethical DisclosuresProtection of persons and animals. The authors declare that no experiments were performed on humans or animals for the purpose of this study.
Data confidentiality. The authors declare that they followed their hospital's regulations regarding the publication of patient information.
Right to privacy and informed consent. The authors declare that no private patient data appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Wortsman X, Millard F, Aranibar L. Ecografía Doppler color en malformaciones glomuvenosas con correlación clínica e histológica. Actas Dermosifiliogr. 2018;109:e17–e21.