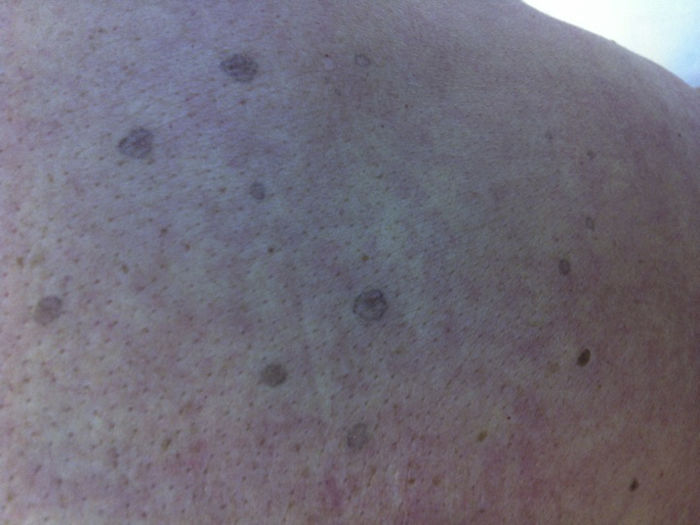
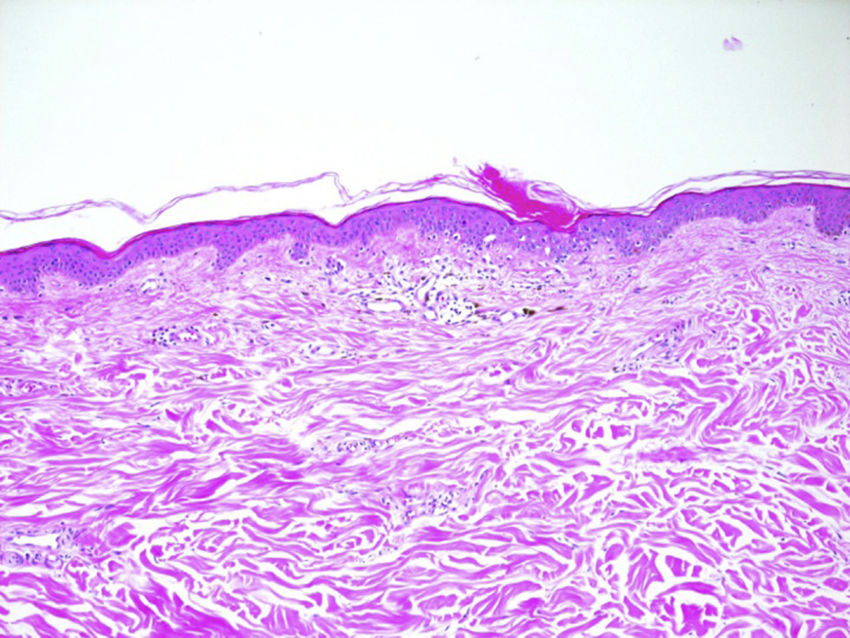
We report a rare skin toxicity in a 62-years-old white female with a history of an estrogen receptor- and progesterone receptor–positive (95% and 50% of tumor cells, respectively) human epidermal growth factor receptor 2 (HER2)–positive invasive ductal carcinoma of the left breast (initial stage pT2 pN1a M0). She underwent radical modified mastectomy plus axillary node dissection followed by adjuvant treatment with 1 year of trastuzumab and 5 years of the aromatase inhibitor letrozole. Six years after the diagnosis, the patient developed bone and internal mammary lymph node metastases treated with exemestane and trastuzumab. Two months after starting treatment, she developed non-painful but slightly itching diffuse skin lesions. She showed multiple, disseminated, brown-grey oval macules, slightly palpable, well delimitated, 2-5mm in diameter, many with collarettes of scale. Lesions were located in both sun-exposed and non-sun-exposed sites, predominantly in the back, trunk and upper extremities (Figure 1). The biopsy of a back lesion revealed a focal thinning of the epidermis, with loss of the granular layer and a discrete column of parakeratosis (Figure 2). Symptomatic treatment with topical bethametason and salicyl acid was initiated with resolution of pruritus. Patient did not accept the proposed systemic treatment with Acitretinon nor Tacalcitol oinment. The patient is still receiving the combined therapy after achieving a complete disease remission and macules persist unchanged without dose reductions or lengthening of treatment intervals. No new skin adverse reactions were observed.
Trastuzumab is a monoclonal antibody that binds to the extracellular domain of HER2 and exemestane is an aromatase inhibitor which suppresses plasma oestrogen levels by inhibition of the enzyme aromatase. Acne vulgaris, nail changes, pruritus, leukopenia and more rarely cellulitis, dermal ulcer and erysipela have been described under trastuzumab treatment. Alopecia, dermatitis, itching, acute generalized exanthematous pustulosis, pruritus and urticaria were reported for exemestane.1
Porokeratosis is a heterogeneous group of hereditary or acquired disorders of keratinization believed to arise from an expansion of abnormal keratinocytes. Multiple variants of porokeratosis have been described.2,3 Recently, a new entity named Eruptive porokeratosis has been reported to describe cases of acute, disseminated eruptions.3 Pathogenesis is not well understood. Some proposed triggering factors include genetics, ultraviolet light exposure, infection, and immunosuppression (ie,transplant recipients, retroviral disease).2,4–6 Some reports of drug-induced cases are also reported, mainly due to immunosuppressing drugs (ie, prednisone, antirheumatic drugs, biologics).3–5 Some authors speculate that porokeratosis, specially the eruptive form, may represent a paraneoplastic manifestation since it has been described in association with hematopoietic malignancies orsolid organ tumors (ie, hepatocellular carcinoma, cholangiocarcinoma, ovarian cancer).3–7 The typical histological feature of porokeratosis is the cornoid lamella, which corresponds to the border between normal epidermis and the clone of mutant keratinocytes. Prognosis of porokeratosis is generally good but some cases of squamos cell carcinoma developed on porokeratosis have been described, suggesting porokeratosis as a possible pre-cancer situation. Proposed treatments are photodinamic therapy, local tacalcitol or Acitretinoin for disseminated variants.2,3
No data have been reported so far about the link between porokeratosis and exemestane or trastuzumab. As the patient is still receiving the same oncological treatment, persistence of the lesions could be linked with either the immunotherapy or the antihormonal therapy. Porokeratosis is a disorder of keratinization and antibodies targeting the HER-family receptors can cause disorders at this level The possibility that eruptive porokeratosis must be considered as a paraneoplastic phenomenon in our case cannot be completely ruled out. Nevertheless, the long period of time between both conditions, the fact that skin eruption persists although tumor response to the treatment and a time association with the treatment starting supports a drug induced phenomenon in our opinion.
Please cite this article as: Mangas C, Espeli V, Blum R. Un caso de poroqueratosis diseminada eruptiva en un paciente oncológico tratado con trastuzumab y exemestano: ¿fenómeno asociado al cáncer o inducido por fármacos?. Actas Dermosifiliogr. 2018;109:559–560.