Vulvar skin disease is a common reason for consultation. The vulva, like the rest of the skin, can be affected by numerous diseases of various etiologies, but its particular anatomic and physiologic characteristics create additional diagnostic and therapeutic difficulties. The study of vulvar disease is emerging as a new branch of dermatology. In this article, we examine the characteristics of the normal vulva, and perform a brief, structured review of vulvar inflammatory dermatoses, which comprise a heterogeneous group of diseases in which a broad, multidisciplinary approach is essential.
La patología cutánea vulvar constituye un motivo frecuente de consulta en el momento actual. La vulva, al igual que el resto de la piel, puede verse afectada por múltiples enfermedades de diferentes etiologías, pero sus especiales condiciones anatómicas y fisiológicas hacen que tenga algunas peculiaridades que pueden suponer una dificultad añadida en su manejo. El estudio de la patología vulvar está emergiendo como una nueva área en la Dermatología. En este artículo, tras valorar la características de la vulva normal, sistematizaremos y realizaremos una breve revisión de las dermatosis inflamatorias vulvares, grupo heterogéneo de enfermedades en las que es clave un abordaje amplio y multidisciplinar.
The female external genitalia is called the vulva. The name derives from the Latin word vulva, meaning sheath, which was used in Latin to refer to the uterus.1 The vulva is a complex and specific topographic area of the skin that varies from one individual to another and comprises several anatomical structures. Its complex morphology and the variety of functions that characterize this mucocutaneous area (the conjunction of the urinary, genital, and gastrointestinal systems) make the vulva susceptible to a wide range of diseases that require a multidisciplinary approach involving several specialties, including gynecology, urology, pathology, and dermatology.2
While vulvar skin disease is a common reason for consultation, fear and cultural taboos lead some women to conceal their condition or fail to consult a physician about their symptoms. Moreover, the lack of experience on the part of some clinicians in identifying vulvar diseases and the “awkwardness” involved in a physical examination of the genitalia have also led to delays in diagnosis and therapy in many cases.
From the point of view of dermatology, the vulva, like the rest of the skin, may be affected by many disorders with differing etiologies. The disorders may be specific to the vulva or have a predilection for this area, or they may form part of a broader clinical picture. Vulvar diseases do, however, have certain peculiarities because the moisture, friction, and occlusion that characterize the area give rise to modifications in the typical clinical features. Symptoms are often vague (burning, stinging, itching) and common to many conditions. Secondary complications, such as infections or lesions caused by scratching, can also make assessment more difficult. The first step is to obtain a thorough medical history. Social implications, fear of malignancy or sexually transmitted diseases, and repercussions on sexual relations and intimacy cause some women to feel isolated and make it difficult for them to explain their symptoms. This can lead patients to postpone consultation, a decision that may result in progress to chronic or advanced disease. A good doctor-patient relationship is essential: the patient must feel able to express herself freely and the physician should explain the nature of her symptoms simply.3 Every effort should be made to avoid the frustration and depression often associated with these disorders.4 Finally, the clinician should have appropriate photographs or a diagram of vulvar anatomy in the consulting room to familiarize the patient with the anatomy and to be used as an aid for describing symptoms; a mirror is also useful for self-exploration.3
Vulvar dermatosis has, for a long time, been considered an orphan disease. However, the study of the vulva is now emerging as a recognized branch of dermatology and there is growing interest in research in the context of a broad multidisciplinary approach in which dermatologists play a key role in the diagnosis and management of this group of disorders.6
Normal Vulvar Anatomy and Anatomical VariationsThe term vulva refers to the external genital organs of the female as a whole. It is defined as the area located within the anterior perineal triangle delimited anteriorly by the mons pubis, posteriorly by the perineum, laterally by the inguinal folds, and medially by the hymenal ring.7 It comprises several anatomical structures, principally the mons pubis, the labia majora and minora, the interlabial sulci, the clitoris, the clitoral hood or prepuce, the vaginal vestibule or introitus (the area between Hart's line and the hymenal ring), the urinary meatus, the greater vestibular glands (Bartholin glands) in the posterior third of the vestibule, and the lesser vestibular glands (Skene glands) situated on either side of the urinary meatus.8 Hart's line is located on the medial aspect of the labia minora and represents the border between the modified mucosa of the labia minora (keratinized epithelium) and the mucosal lining of the vaginal vestibule (transitional nonkeratinized epithelium). The vestibule, which is the innermost portion of the vulva, extends from this line to the hymen. The blood supply of the vulva is derived from the internal and external pudendal arteries. The anterior and superior regions are innervated by the ilioinguinal and genitofemoral nerves (lumbar plexus branches) and the rest by the pudendal nerve. The appearance of the vulva varies greatly depending on age, race, and hormonal factors. Different degrees of pigmentation are seen as well as asymmetry or hypertrophy of the labia minora.5
Of particular note are the 2 physiologic anatomical variations described below.
- 1.
Sebaceous hyperplasia (Fordyce spots) is the most common variation.9 It is found in 75% to 95% of women,5 although there are almost no reports in the literature.10 Sebaceous hyperplasia takes the form of small yellowish papules that vary in size from 1 to 2mm and are found on the medial aspect of the labia minora. They correspond to ectopic sebaceous glands and may be prominent and coalesce. They are asymptomatic and do not require treatment.
- 2.
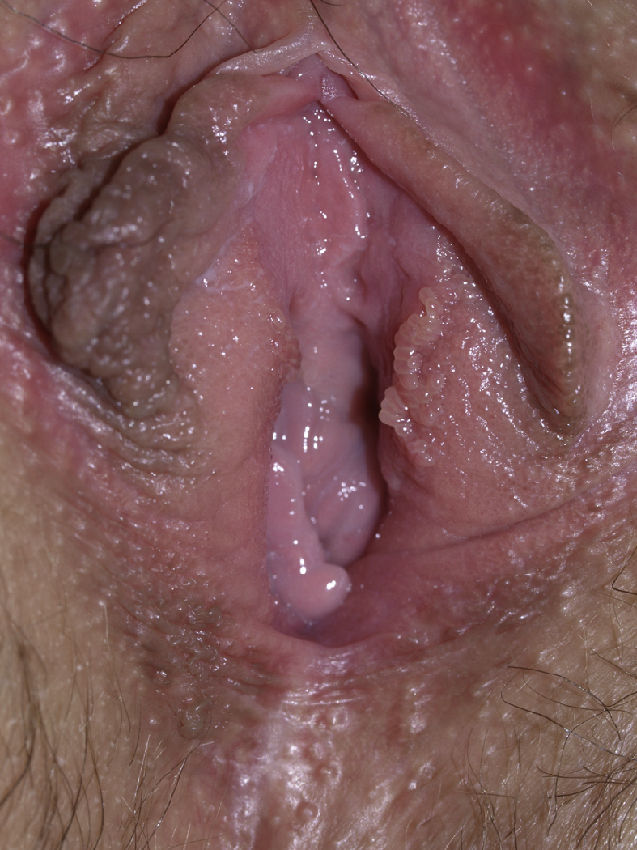
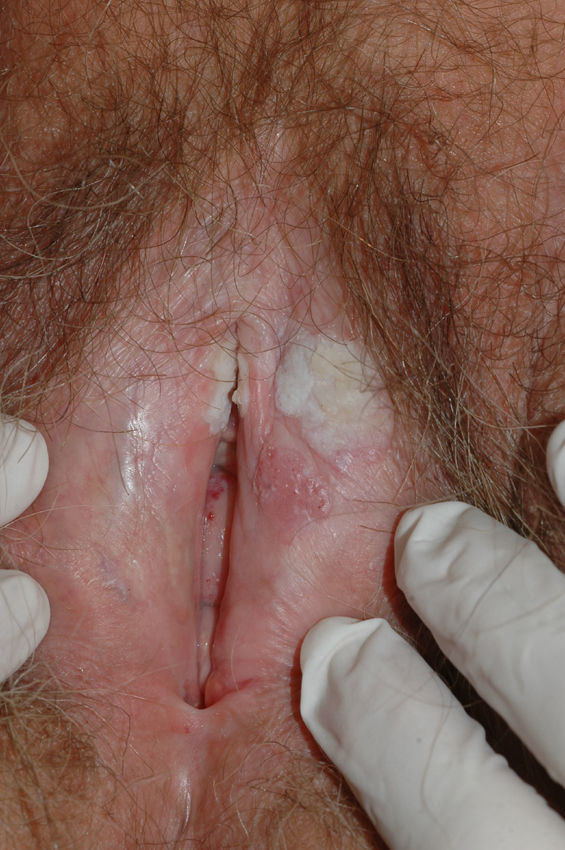
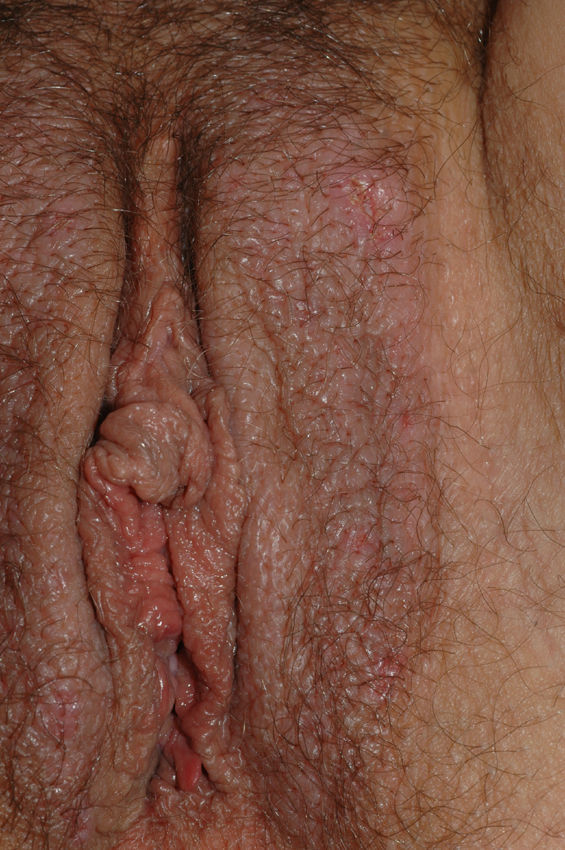
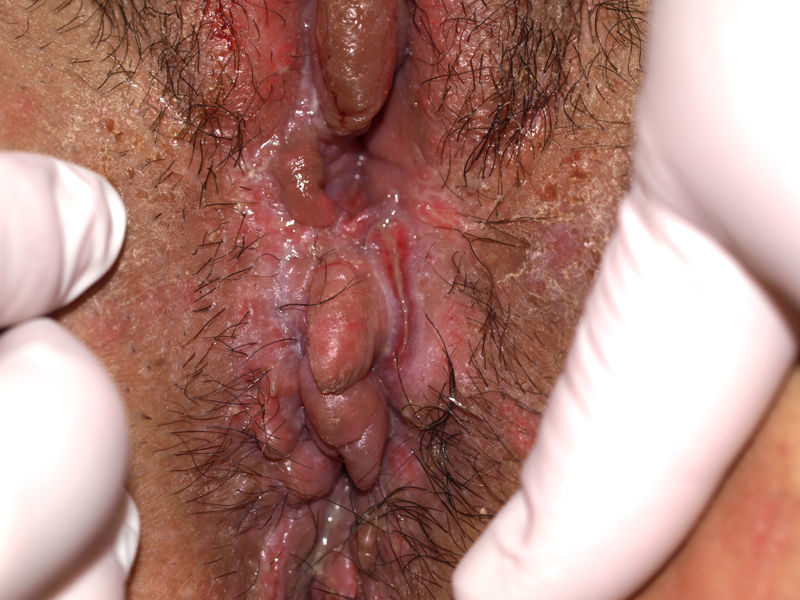
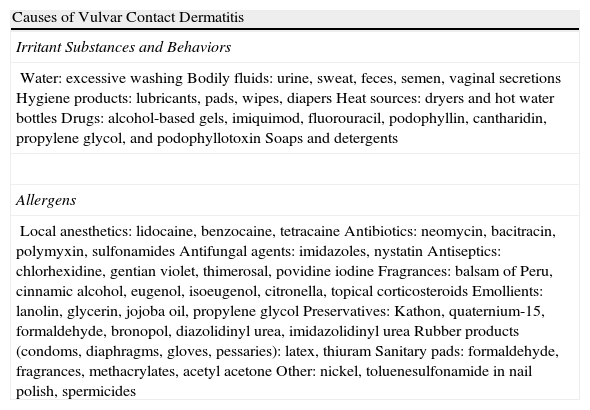
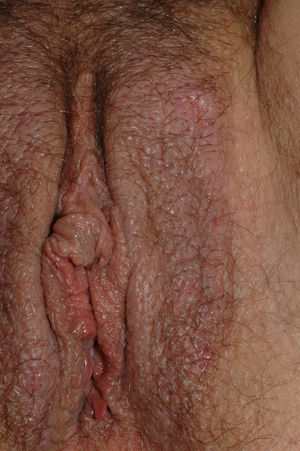
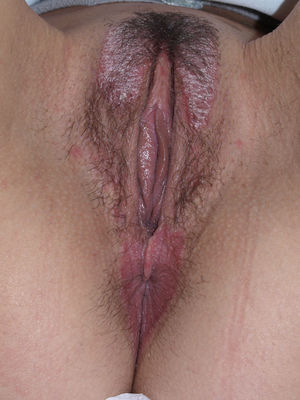
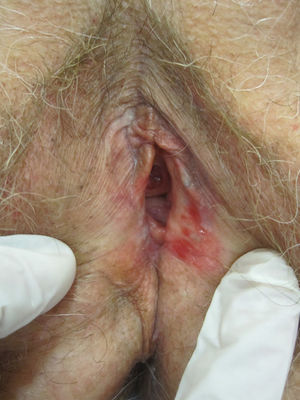
Vulvar or vestibular papillomatosis There is no consensus on the prevalence of this variation. Some studies report prevalence to be 1%,11 while others report rates ranging from 8% to 48%.5 Vulvar papillae were first described in 1981 as pseudocondylomata of the vulva12 and have since been described with several names, including micropapillomatosis, benign squamous papillomatosis, hirsutoid papillomatosis of the vulva, and micropapillomatosis labialis.2 The papillae vary in number and are small, (1-2mm) soft, filiform, monomorphic projections with a color similar to that of the adjacent mucosa. They are distributed symmetrically, primarily in the posterior vestibule (Fig. 1); when the papillae almost entirely cover the vestibule, the condition is known as vulvar papillomatosis.11 Despite initial doubts about the etiology of these projections, the possibility of any relationship with human papillomavirus infection has now been ruled out13 and they are now considered to be a normal variant of the genital epithelium analogous to pearly penile papules.14 It is important to distinguish between these benign projections and condyloma acuminate to avoid inappropriate treatment.15,16 Five clinical parameters have been proposed for identifying vestibular papillomatosis.13 Unlike condylomata, vestibular papillae have a pink color similar to that of the adjacent mucosa. They are soft and their distribution is symmetrical and linear rather than irregular. Each projection has a separate base whereas warts can take the form of clusters of filiform projections on a single base. Finally, they do not blanch when exposed to acetic acid. Dermoscopy is also useful, revealing abundant irregular blood vessels in the almost transparent center of rounded papillae of uniform size on separate bases.17 The papillae are generally asymptomatic, although in some cases they may cause itching, pain, or burning and interfere with normal life and sexual relations.11,18,19 They do not require treatment.
Systematic classification of the diseases that affect the vulvar region is difficult as they are many and varied, ranging from inflammatory dermatoses to infections, tumors, pigmentary alterations, and even chronic pain syndromes, such as vulvodynia. One of the goals of the International Society for the Study of Vulvovaginal Diseases (ISSVD) is to develop classifications and standardize the nomenclature of these diseases. The ISSVD has updated the terminology and classifications of vulvar intraepithelial neoplasia,20 vulvar dermatoses,21 and vulvodynia.22
The present article offers a brief, systematic review of vulvar inflammatory dermatoses, a group of disorders often encountered in clinical practice. The most recent classification is based on the histologic patterns characterizing each entity. Careful correlation of clinical and histologic findings is essential for diagnosis (Table 1).23 This latest classification could be considered to be of little use in the diagnosis and management of these conditions in daily practice because several disorders can occur concomitantly and the clinical pictures of different entities tend to overlap. It is, nonetheless, a useful tool in the study, comparison, and discussion of these diseases among clinicians and in working groups.
Classification of Inflammatory Vulvar Dermatoses.
| Lichenoid Pattern |
| Lichen sclerosus |
| Lichen planus |
| Dermal Sclerosis Pattern |
| Lichen sclerosus |
| Spongiotic Pattern |
| Atopic dermatitis dermatitis |
| Irritant and allergic contact dermatitis |
| Vesiculobullous Pattern |
| Bullous pemphigoid |
| Mucous membrane pemphigoid |
| Gestational pemphigoid |
| Pemphigus vulgaris |
| Linear immunoglobulin A disease |
| Bullous erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis |
| Acanthotic pattern |
| Lichen simplex chronicus |
| Psoriasis |
| Reiter syndrome |
| Acantholytic Pattern |
| Hailey-Hailey disease |
| Darier disease |
| Acantholytic dermatosis of the vulvocrural area |
| Granulomatous Pattern |
| Crohn disease |
| Melkersson-Rosenthal syndrome |
| Vasculopathic Pattern |
| Aphthous ulcers |
| Behçet disease |
| Plasma cell vulvitis |
Adapted from the 2006 ISSVD Classification of Vulvar Dermatoses.21
On the basis of the classification of vulvar dermatosis according to histological pattern, a number of subsets have been defined.23
Lichenoid PatternLichen SclerosusLichen sclerosus is a chronic inflammatory autoimmune skin disease that predominantly affects the anogenital region. It was first identified in the 19th century by Hallopeau24 and Darier,25 who described it as a variant of lichen planus. It has been given several names, including kraurosis vulvae, atrophic lichen sclerosus, and hypoplastic dystrophy. The name currently recommended by the ISSVD is lichen sclerosus.26
While lichen sclerosus is a common condition, it is believed that its prevalence may be underestimated for the following reasons: patients are treated in different specialist areas; in some settings there a lack of experience in recognizing the disorder; patients fail to consult their physician promptly; and onset is asymptomatic in 9% of cases.27–29 Its prevalence has been estimated at between 1:300 to 1:1000 among women consulting clinics specializing in vulvar disease;30 in 1 clinic, women with lichen sclerosus accounted for 39% of all patients seen.31 Other studies have reported a prevalence of 1.7% among women consulting a gynecologist32 and of almost 3% among women living in a nursing home for elderly people.33 Although it has been reported in patients of all age groups and in both sexes, lichen sclerosus is most common among postmenopausal women,26 although it may also appear in childhood (15%). In girls, it typically improves with age but can persist into adulthood.34,35
The exact etiology is unknown and probably multifactorial.36,37 Genetic, autoimmune, hormonal, and infectious factors have all been implicated in its pathogenesis.38,39 The genetic contribution is complex; it is estimated that at least 11% of patients with lichen sclerosus have family members with the condition,28 although the pattern of inheritance is unclear.34 Authors who studied different associations with genes regulating the major histocompatibility complex40,41 found associations with HLA-DQ7 and, to a lesser extent, HLA-DQ8 and DQ9, particularly when onset occurred in childhood.40,42–44 An association between lichen sclerosus and HLA-DRB1 * 12/DQB1 * / 0301/04/09 /010 was recently found. The HLA DRB1*0301 /04 and DRB1*0301/04/DQB1*0201 /02/03 haplotypes, however, appear to protect against the risk of lichen sclerosus.45 Moreover, it is believed to be an autoimmune disease in view of the association with other autoimmune diseases, such as thyroiditis,46 alopecia areata, pernicious anemia, and vitiligo,47–49 and the high incidence of autoantibodies and family history of autoimmune diseases observed in these patients. Since the initial inflammation in lichen sclerosus affects the basement membrane, it has been suggested that the target antigen must be in this region. Evidence has been found of circulating autoantibodies against protein 1 of the extracellular matrix (ECM-1) in 67% of cases,50 and of antibodies against basement membrane regions (mainly BP180 and BP230) in 30% of cases.50–52 A recent study showed that in over 40% of patients with vulvar lichen sclerosus and lichen planus, the NC16A domain of BP180 was a target for circulating T cells and that associated autoantibodies against BP180 were present.53 Debate also continues regarding the etiologic and pathogenic role of Borrelia burgdorferi, since evidence of the involvement of this bacteria has been found in Europe although not in the United States.54 Finally, a possible hormonal mechanism has been posited because the disease tends to appear during periods of life when the influence of estrogen is lower.42
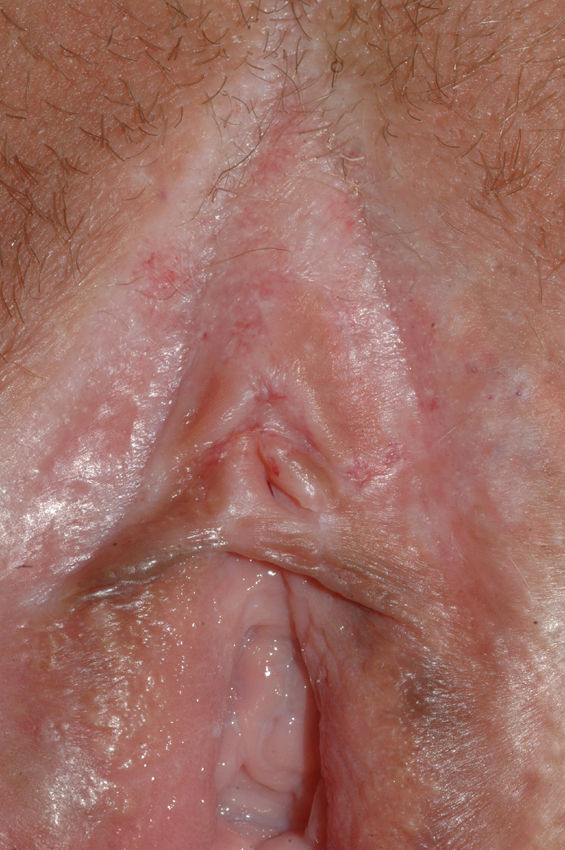
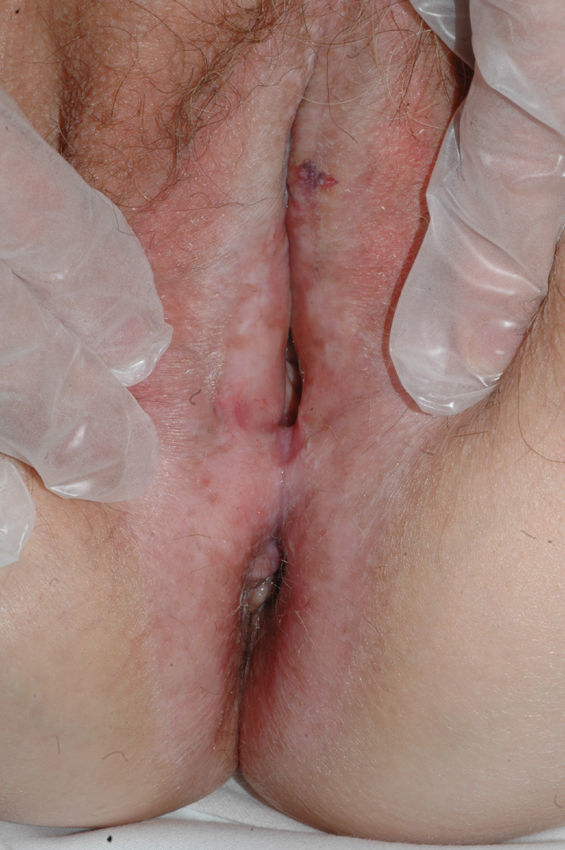
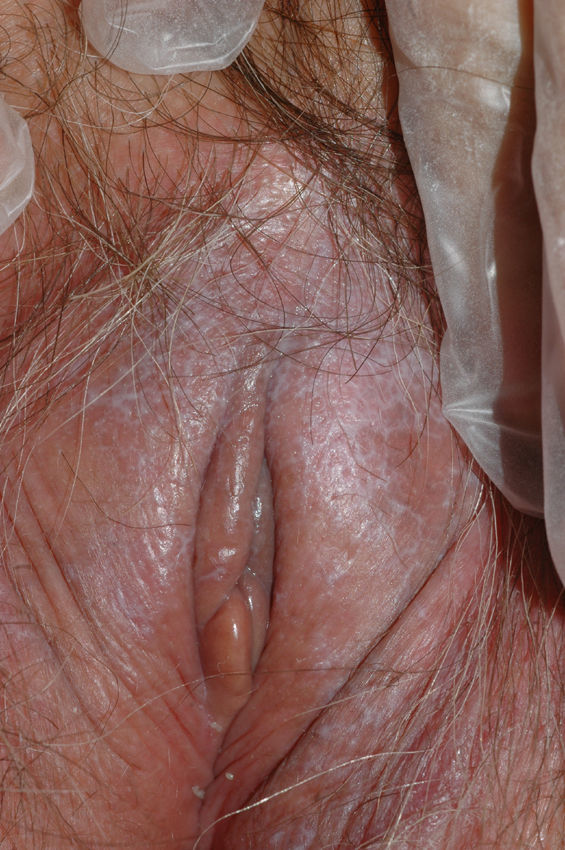
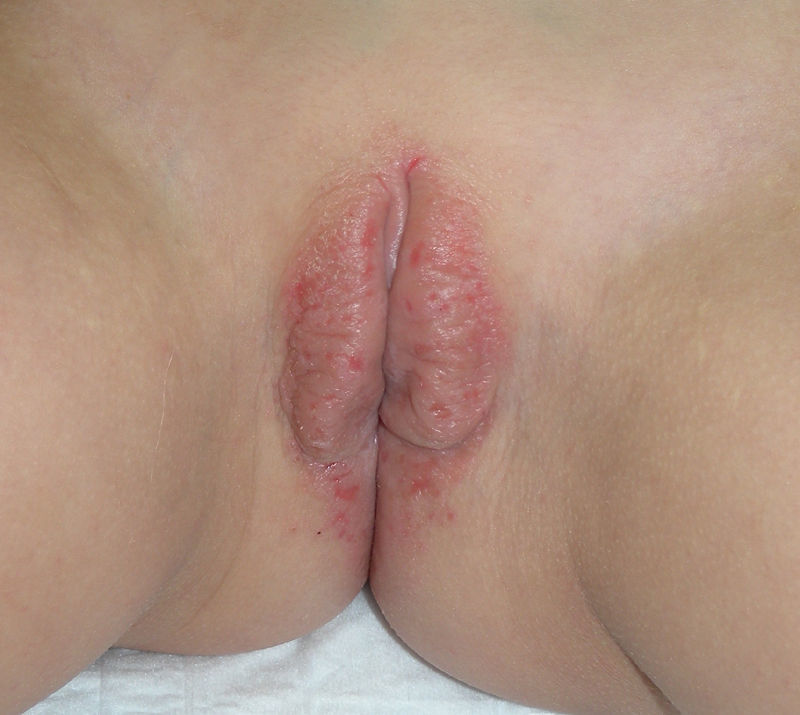
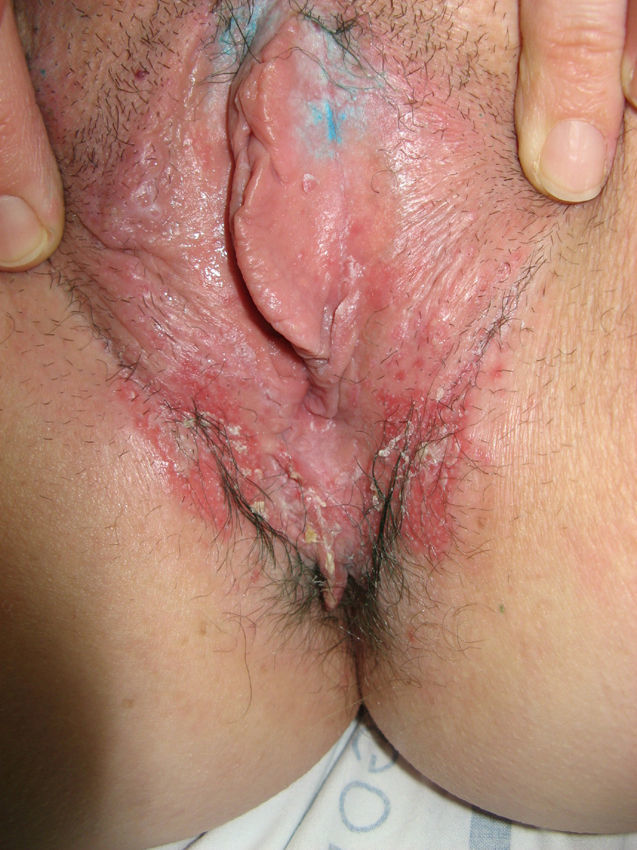
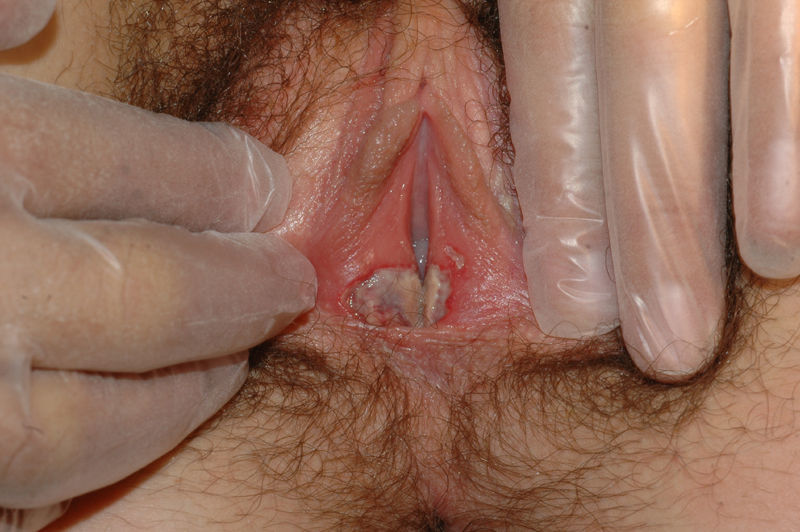
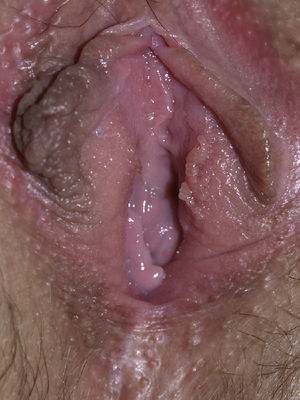
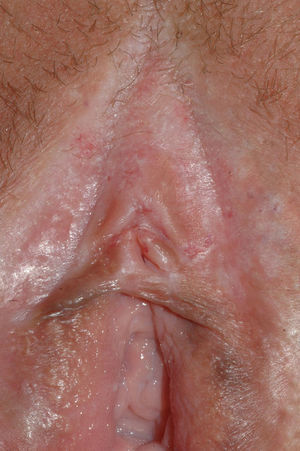
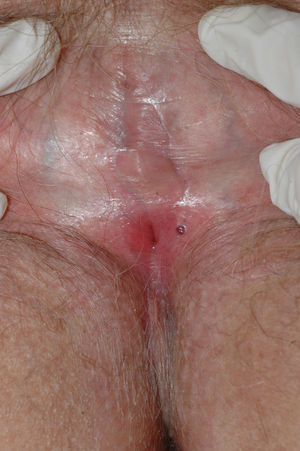
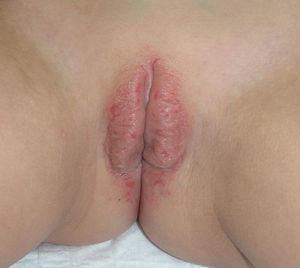
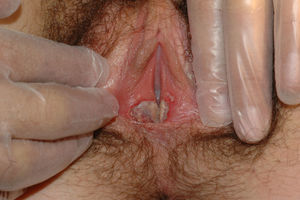
The most common symptom is pruritus, although some patients experience pain, dyspareunia, dysuria, constipation, or secondary infections. The disease is asymptomatic in 1% of cases.41 The symptoms vary considerably depending on the site and the stage of development. Initially, lichen sclerosus usually affects the area around the clitoris (Fig. 2) and in advanced cases spreads to form the typical figure-of-eight pattern around the vulvar and perianal area, which is affected in 60% of cases (Fig. 3).41 Extragenital manifestations are present in 6% of cases,47 but the oral and genital mucosa are not usually affected. Only 1 case of vaginal lichen sclerosus has been reported.55
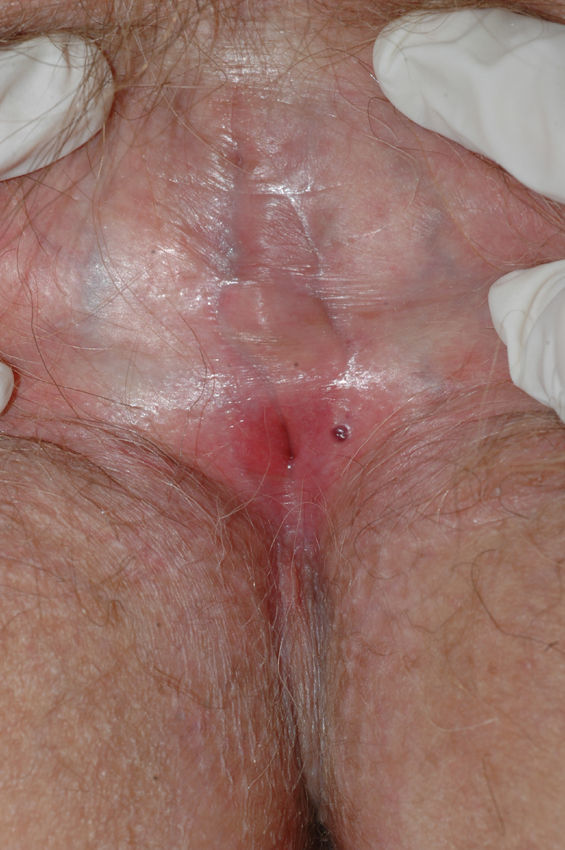
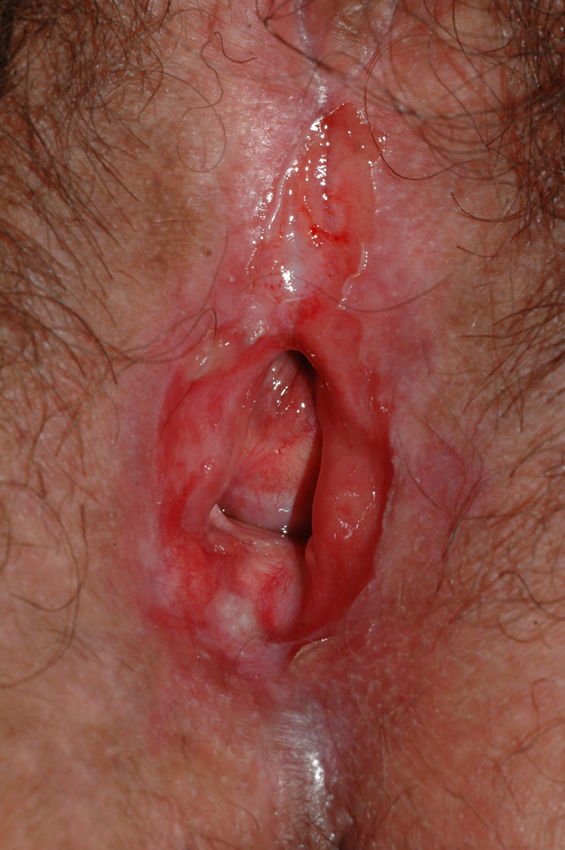
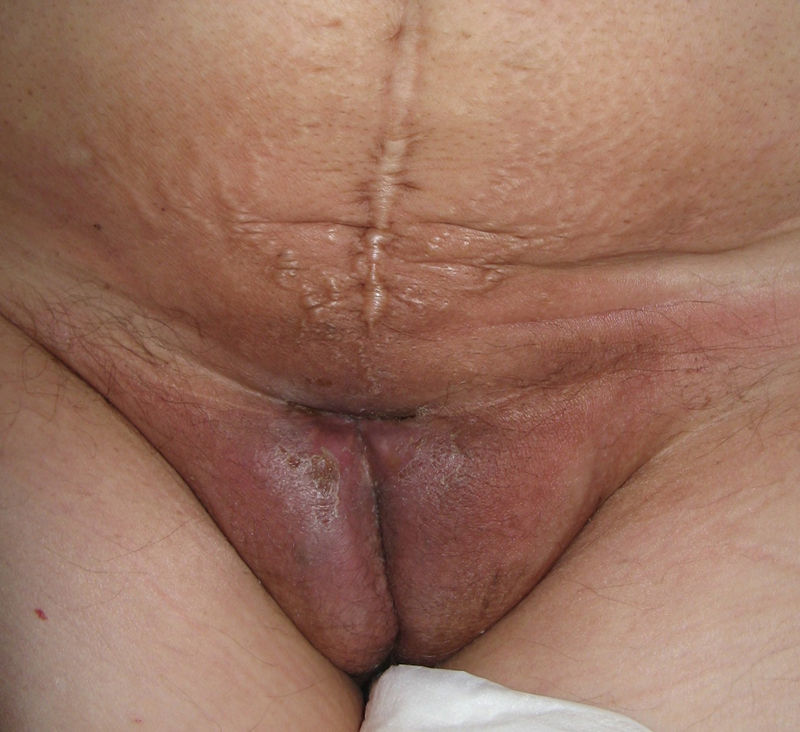
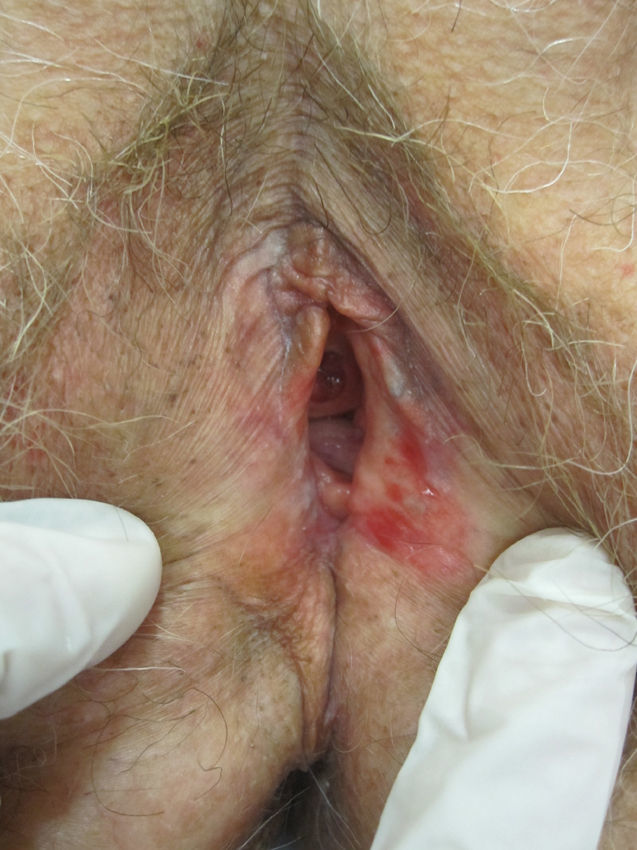
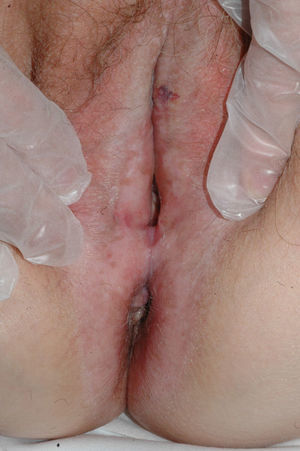
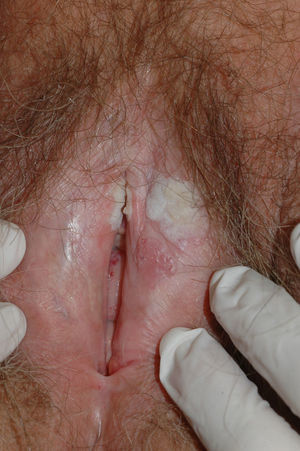
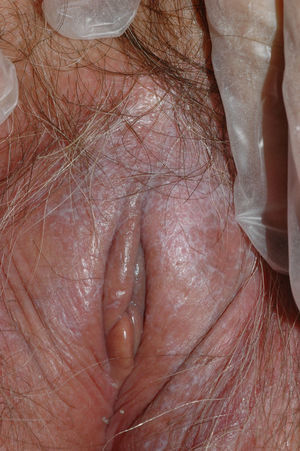
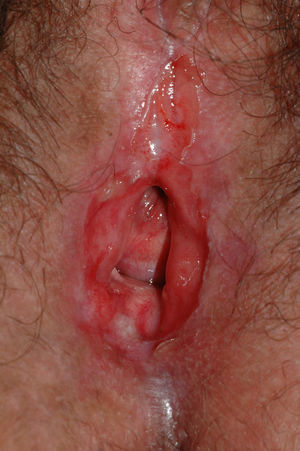
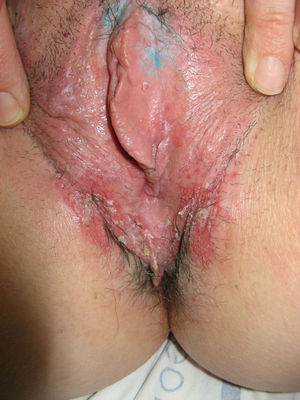
Lichen sclerosus is characterized by the presence of well-defined pearly white papules and plaques accompanied by a characteristic alteration in skin texture in that skin becomes extremely thin and fragile. This fragility may result in the presence of purpura, erosions, and fissures. Itching may lead to lesions caused by scratching and lichenification that can complicate diagnosis. In advanced cases, patients present with differing degrees of sclerosis, and scarring can result in architectural destruction with resorption of anatomical structures: obliteration or synechiae of the labia minora and clitoris, complete loss of the labia minora, and in severe untreated cases even stenosis of the vaginal introitus (Fig. 4). Lichen sclerosus is sometimes associated with patchy hyperpigmentation of the mucosa and vestibule. Lichen sclerosus is associated with squamous cell carcinoma of the vulva25 and, more rarely, verrucous carcinoma (Fig. 5).56 The risk of this association is estimated to be between 2% and 5%.41,57–59 Risk is thought to be higher in patients in whom diseases is poorly controlled.58 The diagnosis of lichen sclerosus is usually clinical, and the differential diagnosis includes psoriasis (a condition with which it is sometimes associated60), lichen planus, lichen simplex chronicus, and mucous membrane pemphigoid. While not always necessary, skin biopsy is advisable to confirm the diagnosis, especially when the patient presents scarring lesions and loss of vulvar architecture.38 Biopsy is essential in the case of a doubtful diagnosis and in atypical cases, when malignancy is suspected (persistent hyperkeratosis or erosion), when disease fails to respond to standard treatments, and when pigmented areas or extragenital lesions suggestive of morphea are observed.27 In certain cases it may be useful to screen for autoantibodies,27 although the need for this is debated and such testing is not generally recommended.
Histologic findings will vary depending on when the biopsy is performed and the site where the specimen is obtained. Early changes include basal layer vacuolization, occasional keratinocyte necrosis, and a bandlike lymphohistiocytic infiltrate in the superficial dermis. More stable lesions show an atrophic epidermis and edema in the papillary dermis together with sclerosis and hyalinization of collagen bundles, an alteration that gives the superficial dermis a homogenized appearance. Parakeratosis and epidermal hyperplasia are sometimes observed ssociated with lichen simplex.23
The aim of treatment is to reduce itching and other symptoms, improve the patient's quality of life, and reduce skin lesions as well as to prevent disease progression and possible malignant transformation to squamous cell carcinoma.39 Patients should be given a clear explanation of their condition, including the long-term implications and the treatment options available. General advice on hygiene is essential; patients should be advised to avoid irritants, to use unscented soaps and emollients, to wear cotton underwear, and to apply lubricants and emollients when necessary.26 Although different therapies have been proposed, none has proved more effective than very potent topical corticosteroids (clobetasol propionate 0.05%), which are therefore the treatment of choice.27 Topical corticosteroid therapy has not been studied in randomized controlled trials for this indication and a number of regimens have been proposed.27,41 Frequency of application should be based on the appearance of the skin and not on the symptoms.37 While some authors report complete remission in around 60% of patients,41,61 other studies have reported partial or complete remission in up to 95% of women who apply this treatment daily for 3 months.62 The labia minora and the region around the clitoris are resistant to the adverse effects of topical corticosteroids (atrophy, telangiectasia), making prolonged maintenance regimens safe.63 When response to other treatments is poor or the associated side effects unacceptable, topical treatment with calcineurin inhibitors (pimecrolimus and tacrolimus) has also been shown to be useful, with a beneficial effect being reported in nearly 50% of patients.27,64 However, these drugs are a second-line treatment because of the lack of long-term studies, the possible irritation they may cause, and the controversy about their use in a disease with potential for malignant change. Testosterone and other hormone treatments have been used in the past, but there is currently no evidence supporting their use.27 The possible usefulness of systemic therapy (ciclosporin, methotrexate, and other immunosuppressants) has been reported for cases of treatment-resistant disease. Surgery is only indicated for malignant disease or cicatricial sequelae such as vaginal stenosis. In the case of stenosis, surgery should be performed after the resolution of inflammation, and further treatment is essential to prevent recurrence.54
Lichen PlanusLichen planus is an inflammatory skin disease that can affect any area of the skin or mucous membranes. The presentation varies depending on severity, the stage of development of the lesions, and the site affected.65 The etiology is poorly understood, although it is thought to be a T cell–mediated autoimmune disease because of its association with other autoimmune diseases and its response to immunosuppressive therapy.49,53,66,67
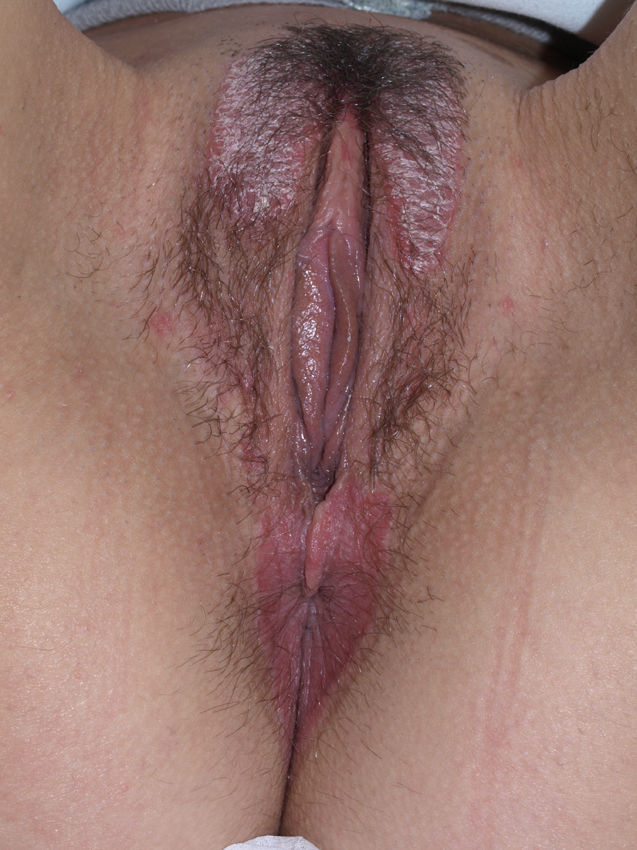
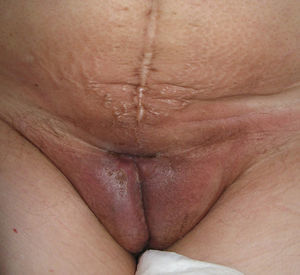
Lichen planus may be localized to the vulvovaginal area or it may affect this area in the context of more extensive disease. It is estimated that the vulva is affected in approximately 50% of women with oral lichen planus,54,65,68 although some authors have suggested that such involvement may be underdiagnosed.69–71 Some two-thirds of patients with vulvar involvement also have vaginal and gingival disease, a triad known as vulvovaginal-gingival syndrome.72–75 Vulvovaginal lichen planus can take different clinical forms, but given the nature of the anogenital mucosa, the most common findings are intense erythema affecting the introitus and vagina, whitish striae (Fig. 6), and whitish epithelium. Well-defined and intensely erythematous ulcers or erosions appear in the variant known as erosive lichen planus, which is more common in the vulvar area, with a reported presence in between 74%76 and 95%5 of cases (Fig. 7). Lichen planus is usually symptomatic, characterized by pain, burning, or itching, and associated with dysuria, dyspareunia, and postcoital bleeding.65,69,75–77 Other characteristic findings include cicatricial sequelae and synechiae with clitoral burying and possible narrowing of the introitus, vaginal involvement (50%-60% of cases) with or without associated desquamative vaginitis, and in some cases, involvement of other mucous membranes, such as the rectal or esophageal mucosa.5 Like other chronic erosive diseases, it is associated with a 2% to 3% risk of malignant transformation to squamous cell carcinoma.63,75,78,79 Clinical diagnosis is complex, and lichen planus should always be suspected in the presence of intensely red and painful vulvar erosions. It is essential to examine other potential sites. Biopsy may be necessary, although a conclusive diagnosis is obtained from only 70% of biopsies in this setting.68 The specimen should be taken from a whitish area or the edge of an erosion. Characteristic findings include the presence of a bandlike intense lymphocytic inflammatory infiltrate in the dermis with basal layer degeneration and Civatte bodies.23 In addition to lichen sclerosis, lichenoid drug reactions, and plasma cell vulvitis, the differential diagnosis should include blistering diseases such as mucous membrane pemphigoid, pemphigus vulgaris, fixed eruptions, and erythema multiforme. Unlike lichen sclerosus, lichen planus tends to be resistant to treatment and difficult to control.69 Topical corticosteroids are the first-line treatment75 and should be prescribed on a case-by-case basis according to symptoms. Existing recommendations for treatment are based on case series data as no controlled trials have been carried out. There is evidence in the literature indicating a good response to topical calcineurin inhibitors.80,81 A number of systemic treatments (oral corticosteroids, hydroxychloroquine, methotrexate, ciclosporin, retinoids, mycophenolate, cyclophosphamide, azathioprine, etanercept, and infliximab) have been used in cases of treatment-resistant disease with varying results. In cases of vaginal involvement, intravaginal corticosteroid suppositories are useful combined with oral fluconazole (150mg/wk) to minimize the risk of candidiasis.82 Surgery is only indicated when there is stenosis or obliteration. Long-term follow-up is recommended.78
Dermal Sclerosis PatternLichen sclerosus, which has been described above, also falls into this category.
Spongiotic PatternDermatitis is a skin disease that frequently affects the vulvar region. It is characterized by the presence of pruritus, diffuse erythematous lesions, epithelial disruption, and lichenifcation.83 Diagnosis may be complicated by the maceration of the area and by lesions caused by scratching. There are 2 types of dermatitis: endogenous forms, which include atopic dermatitis and lichen simplex, and exogenous or contact dermatitis. Since the histologic features and symptoms of these two entities overlap and both types may even coexist, a thorough medical history followed by careful correlation of histologic and clinical findings is vital for diagnosis. Histologically, dermatitis is characterized by the presence of epidermal spongiosis and a lymphocytic infiltrate in the dermis with occasional eosinophils. Furthermore, the following features may be observed depending on when the biopsy is obtained: in the acute phase, intraepidermal vesicles; in the subacute phase, hyperkeratosis; and in the chronic phase, epidermal hyperplasia.23
Endogenous DermatitisAtopic DermatitisAtopic dermatitis is the most common form of endogenous dermatitis. It generally begins in childhood (Fig. 8) and affects individuals with a personal or family history of atopy. Sometimes the only signs are xerosis, desquamation, and lichenification. The histological changes are not specific.23
Lichen Simplex ChronicusLichen simplex chronicus is one of the most common causes of primary vulvar itching, although this symptom is also caused by other pruritic skin diseases. Primary lichen simplex chronicus is a localized chronic type of atopic dermatitis.84 Constant itching and scratching result in thickening of the skin and damage to the protective barrier layer, leading to irritation, increased sensitivity to exogenous substances, superinfection, and perpetuation of the itch-scratch cycle.85 Clinical manifestations include thickened and lichenified plaques and a unilateral or bilateral increase in skin folds (Fig. 9). Erosions, excoriation, and residual hyperpigmentation may also be observed, particularly in the labia majora. Pubic hair may be affected and other eczematous lesions or other stigmata of atopy may be observed. Diagnosis is clinical, but a biopsy can be useful when diagnosis is in doubt. Histologic features include hyperkeratosis, hypergranulosis, acanthosis, spongiosis, and a chronic inflammatory infiltrate.23
The aim of treatment is to reduce inflammation (potent topical corticosteroids), break the itch-scratch cycle (nighttime antihistamines), and improve barrier function (lubricants), while avoiding irritants and treating possible superinfection.85,86
Contact DermatitisContact dermatitis of the vulva is very common and may complicate other skin diseases, either because of its symptoms or the treatments prescribed.5,83 The condition can be irritant or allergic, and the vulva is considered to be particularly susceptible because the skin is more fragile in this area than in other parts of the body.87,88
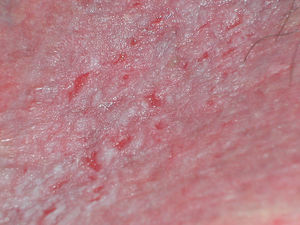
Irritant Contact DermatitisIrritant contact dermatitis is the most common form of contact dermatitis, although its prevalence has not been established. The symptoms are itching, stinging, and pain. The condition is the result of contact with substances that are cytotoxic to keratinocytes without the need for prior sensitization. There are many possible vulvar irritants, including, of particular interest, bodily fluids that may come into contact with this area (urine, feces, semen, sweat), topical medications (Fig. 10), and intimate hygiene products.87 Two common types of irritant dermatitis affect the vulva, one similar to diaper rash (found particularly in older women with urinary incontinence) and the other found in women who engage in excessive personal hygiene (Fig. 11).
Allergic contact dermatitis is less common, but must be considered in the differential diagnosis of skin conditions that do not respond to treatment.89 It is a delayed type IV hypersensitivity reaction that requires prior sensitization. The most commonly identified allergens are fragrances, topical antibiotics, nickel, preservatives, and topical anesthetics.5 Contact allergy tests are essential for diagnosis. The differential diagnosis used depends on the phase. In the acute phase, it is necessary to rule out other vesicular-erosive dermatoses, such as herpes simplex, candidiasis, erythema multiforme, fixed eruption, bullous autoimmune diseases, and erosive lichen planus. In the chronic phase, it must be differentiated from other types of atopic and seborrheic dermatitis), flexural psoriasis, lichen simplex chronicus, and Paget disease.87
In both cases it is essential to avoid the allergen or irritant and eliminate excessively irritant hygiene practices. A list of potential allergens and irritants is shown in Table 2. Depending on severity, the recommended treatment is topical or oral corticosteroids or oral antihistamines and the presence of secondary infection must be ruled out.87
The Most Common Irritants and Allergens in Vulvar Contact Dermatitis.
| Causes of Vulvar Contact Dermatitis |
| Irritant Substances and Behaviors |
| Water: excessive washingBodily fluids: urine, sweat, feces, semen, vaginal secretionsHygiene products: lubricants, pads, wipes, diapersHeat sources: dryers and hot water bottlesDrugs: alcohol-based gels, imiquimod, fluorouracil, podophyllin, cantharidin, propylene glycol, and podophyllotoxinSoaps and detergents |
| Allergens |
| Local anesthetics: lidocaine, benzocaine, tetracaineAntibiotics: neomycin, bacitracin, polymyxin, sulfonamidesAntifungal agents: imidazoles, nystatinAntiseptics: chlorhexidine, gentian violet, thimerosal, povidine iodineFragrances: balsam of Peru, cinnamic alcohol, eugenol, isoeugenol, citronella, topical corticosteroidsEmollients: lanolin, glycerin, jojoba oil, propylene glycolPreservatives: Kathon, quaternium-15, formaldehyde, bronopol, diazolidinyl urea, imidazolidinyl ureaRubber products (condoms, diaphragms, gloves, pessaries): latex, thiuramSanitary pads: formaldehyde, fragrances, methacrylates, acetyl acetoneOther: nickel, toluenesulfonamide in nail polish, spermicides |
The symptoms of lichen simplex chronicus are described above under the heading of spongiotic pattern inflammatory dermatosis. This disorder is also included in this section because significant acanthosis may be found in the biopsy specimen.23
PsoriasisPsoriasis is a chronic multifactorial skin disease that affects 5% of women who present with vulvar symptoms.23 Symptoms include intense itching, pain, and fissures but the condition may also be asymptomatic. Several clinical forms of the disease can affect the vulva (the flexural and pustular forms as well as psoriasis vulgaris), and vulvar involvement is usually accompanied by lesions in other areas. The lesions have characteristic, well-defined borders.90 Psoriasis has a predilection for hairy skin (the labia majora and mons pubis), although the inguinal folds and the inner aspect of the thighs are also affected in the flexural form (Fig. 12). The Koebner phenomenon is common in the vulva because of the continuous friction and exposure to irritants. Diagnosis is based on clinical findings, and biopsy is only required in atypical forms. Suspected psoriasis must be differentiated from seborrheic dermatitis (rare in the vulva), lichen simplex, contact dermatitis, candidiasis, and tinea cruris. Vulvar pruritus and burning sensation in women with psoriasis should be promptly assessed and treated because vulvar involvement has been correlated with symptoms of depression in this setting.91 The condition is usually controlled with topical treatment and avoidance of external irritants.
Reiter SyndromeReiter syndrome is a multisystem disease characterized by urethritis, conjunctivitis, and arthritis. Cutaneous manifestations, fever, and malaise may also be observed. Lesions typically appear on hands, feet, and genitals and are clinically and histologically indistinguishable from those of pustular psoriasis.90 The condition is almost exclusive to males, and vulvar involvement is rare, occurring primarily in women who are seropositive for human immunodeficiency virus.92
Vesiculobullous PatternThe vulva can be affected in a number of blistering skin diseases. Vulvar involvement has been reported in pemphigus vulgaris, bullous pemphigoid, gestational pemphigoid, mucous membrane pemphigoid, dermatitis herpetiformis, epidermolysis bullosa, erythema multiforme, Stevens-Johnson syndrome, and bullous systemic lupus erythematosus.2,93 The initial lesion may be a blister, but this usually gives rise to erosions because of the friction characteristic of the area.94 The forms described below are of particular interest.
Bullous PemphigoidBullous pemphigoid is an autoimmune blistering disease characterized by tense blisters on the skin, sometimes preceded by eczematous or urticarial lesions. Clear or hemorrhagic blisters are observed on the vulva; these lead to the formation of erosions that heal without scarring. The condition tends to affect elderly patients and is usually pruritic. However, vulvar involvement, while rare, is more frequent in childhood, when it can occur either as an isolated localized variant95–100 or in the context of more generalized disease.93 It is important to identify this disease because it is essential to differentiate it from sexual abuse.2,101,102 Diagnosis is confirmed by biopsy. Histologically, bullous pemphigoid is characterized by subepidermal blisters and a dermal inflammatory infiltrate that usually contains eosinophils. Direct immunofluorescence of perilesional skin reveals deposition of immunoglobulin (Ig) G and C3 along the basement membrane. Treatment depends on severity and includes topical and oral corticosteroids, minocycline, niacinamide, dapsone, azathioprine, and cyclophosphamide.
Mucous Membrane PemphigoidMucous membrane pemphigoid (formerly cicatricial pemphigoid103) is a group of autoimmune blistering diseases mediated by autoantibodies against different proteins of the dermoepidermal junction. It primarily affects mucous membranes, and to a lesser degree the skin.104 The oral and conjunctival mucosa are the most commonly involved mucous membranes. The vagina and vulva can also be affected in 17% to 54% of cases.94,105 Onset usually occurs between 60 and 80 years, although cases have been reported in girls with exclusively genital involvement.99,106,107 Intact vesicles are rarely observed. Nonspecific erythema and erosions are found, with the possible appearance at a later stage of scarring, resorption of the clitoris, and gradual narrowing of the introitus. Patients complain of irritation, burning, itching, pain, and dryness. Vulvar involvement may be accompanied by oral and cutaneous involvment.105 It is important to rule out conjunctival involvement because of the risk of blindness. Histologic examination reveals subepidermal blisters with a mixed infiltrate including eosinophils. Plasma cells and fibrosis associated with cicatricial changes may be found in the vaginal mucosa. Direct immunofluorescence shows a linear deposition of IgG and C3 along the basement membrane; IgA and IgM may or may not be present.23 The differential diagnosis includes pemphigus vulgaris, erosive lichen planus,108 erythema multiforme, and lichen sclerosus.109 Treatment will depend on the severity of the case and the site of disease, but its aim is to reduce inflammation and prevent sequelae. Genital involvement is considered high risk because it can lead to scar formation, vaginal adhesions, and stenosis, and therefore requires aggressive multidisciplinary treatment.110 A number of systemic treatments have been used, including oral prednisone with cyclophosphamide, azathioprine, mycophenolate, ciclosporin, methotrexate, dapsone, intravenous immunoglobulins,111 etanercept,112 and infliximab; the results have been mixed.
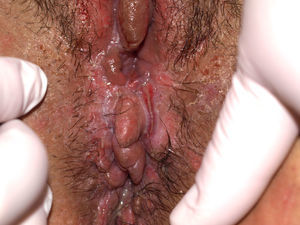
Pemphigus VulgarisPemphigus vulgaris is an autoimmune blistering disease of the skin and mucous membranes characterized histologically by the presence of suprabasal acantholysis and immunologically by the presence of IgG antibodies against keratinocyte cell surfaces (antidesmoglein 3). The prevalence of vulvar involvement is unknown, although a recent study estimated that the vulva is affected in 51% of patients with pemphigus vulgaris.113 The disease presents in the form of painful irregularly-shaped erosions of variable size (Fig. 13) located primarily on the labia minora. However, it can also affect the vagina (36%) and cervix (15%) and may in some cases even be localized to these areas where, unlike other sites, lesions may leave residual scarring.114 The differential diagnosis should include lichen planus, bullous erythema multiforme, and mucous membrane pemphigoid. A biopsy is required to establish diagnosis. Histologically, the disease is characterized by acantholysis and intraepidermal blisters with a row of basal cells in palisade on the floor of the blister. Direct immunofluorescence reveals intercellular deposition of IgG and C3.23 In certain cases, a complete gynecological examination, and even cervical cytology, is recommended.113 It should be noted that patients with pemphigus vulgaris may reveal acantholytic suprabasal cells with dyskaryotic changes indistinguishable from cervical intraepithelial neoplasia, making necessary a more complete differential diagnosis between these 2 entities.114 In cases of localized disease, treatment with topical corticosteroids is recommended. When involvement is extensive, other treatments should be considered, including systemic corticosteroids and immunosuppressants such as dapsone, azathioprine, methotrexate, cyclophosphamide, mycophenolate, intravenous immunoglobulins, and rituximab.93,115
Linear IgA Bullous DermatosisLinear IgA bullous dermatosis is a vesicular-bullous disorder characterized clinically by the presence of polycyclic or annular erythematous plaques surrounded by a single row of vesicles, a pattern that has been called the string of beads sign. This condition affects the mucous membranes, and specifically the genitalia, in a manner almost indistinguishable from that of mucous membrane pemphigoid. Histologic findings are not specific and direct immunofluorescence of perilesional skin is required in which linear deposition of IgA along the basal membrane basal will be observed.23
Acantholytic PatternHailey-Hailey Disease (Familial Benign Pemphigus)Hailey-Hailey disease is an autosomal dominant acantholytic genodermatosis resulting from a mutation in the ATP2C1 gene. Symptoms usually appear in adolescence, particularly in flexures and intertriginous zones. Localized vulvar involvement has been reported.116,117 The disease presents as pruritic linear and angular erosions with whitish maceration and a fetid odor. Superinfection is frequent. Histologic findings include intraepidermal acantholysis in which the cells fall apart giving rise to a dilapidated brick wall appearance. The results of direct immunofluorescence are negative.
Darier DiseaseDarier disease is an autosomal dominant genodermatosis with variable expression caused by a mutation on chromosome 12. It takes the form of hyperkeratotic papules in seborrheic regions, and it may affect the vulva. Superinfection is common.83 Histologic examination reveals columns of parakeratosis, epidermal acanthosis, and suprabasal acantholysis. Other histologic features include epidermal dyskeratosis with corps ronds and grains.23
Acantholytic Dermatosis of the Vulvocrural AreaAcantholytic dermatosis of the genitocrural area is a rare disorder that falls into the spectrum of focal acantholytic dyskeratosis. It was first described as an independent entity by Chorzelski and colleagues118 in 1984. Clinically, it presents as skin-colored, whitish or slightly erythematous solitary or multiple papules or plaques preferentially located on the labia majora, although lesions may also be found in the perineum, the groin, and on the superomedial aspect of the thighs.119 The lesions may be painful or pruritic. The etiology and pathogenesis are poorly understood. Histologic findings are similar to those of Hailey-Hailey disease and Darier disease; prominent acantholysis is observed, which may involve the full thickness of the epidermis, as well as dyskeratosis with grains and corps ronds. Hyperkeratosis and focal parakeratosis may also be observed. The differential diagnosis includes Darier disease, Hailey-Hailey disease, pemphigus vegetans, and warty dyskeratoma.119 Treatment is complicated. Solitary lesions can be treated by surgical excision,120 but multiple lesions tend to persist and are refractory to numerous therapies, although topical tretinoin proved useful in 1 case.119
Granulomatous PatternCrohn DiseaseCrohn disease is an inflammatory bowel disease characterized by the presence of noncaseating granulomas. Mucocutaneous involvement is common, occurring in 20% to 40% of cases,121 although more recent studies report a rate of 15%.122 Genital involvement (70% of patients with cutaneous symptoms)123 is more common in children and is usually the result of direct spread of perineal disease. More rarely, it may represent metastatic spread of Crohn disease.23 Vulvar symptoms occur in 2% of women with Crohn disease,124 with the most common being erythema and asymmetrical labial edema. Other manifestations include bilateral edema, erosions, linear ulceration in the interlabial suci or inguinal folds, abscesses, vulvar fistulas, and even scarring (Fig. 14).125–127 There have also been rare reports of pyoderma gangrenosum or squamous cell carcinoma developing on these lesions.124 Histologic examination may reveal the presence of noncaseating granulomas in the dermis, making necessary differential diagnosis with hidradenitis suppurativa. Specific staining techniques should be used to rule out infections.23 As cutaneous alterations precede intestinal symptoms in 20% of cases, clinical suspicion is important, especially in children with genital lesions and in patients with linear knife-cut ulcers in folds, a sign that is almost pathognomonic of this disorder.126 The first-line treatment for the vulvar form of Crohn disease is oral metronidazole, which is not necessarily the treatment of choice in patients with bowel disease. Ciprofloxacin can also be used, either alone or in combination with oral metronidazole. In refractory cases or in the presence of fistulas, anti-tumor necrosis factor agents such as infliximab, etanercept, and adalimumab are also used.122
Granulomatous VulvitisGranulomatous vulvitis is a rare entity of unknown etiology characterized by the presence of a chronic granulomatous inflammatory infiltrate, similar to and indistinguishable from that observed in Crohn disease and sarcoidosis.128 There is some controversy surrounding the nomenclature of this entity and it has been called by other names, including hypertrophic vulvitis, idiopathic granulomatous vulvitis, chronic edema of the vulva, and Melkersson-Rosenthal vulvitis. It is currently considered to be a subtype of anogenital granulomatosis, a term analogous to orofacial granulomatosisthat that emerged to encompass the spectrum of disorders characterized by persistent labial edema and the presence on biopsy of nonnecrotizing epithelioid cell granulomas in the deep dermis, edema, and a lymphocytic infiltrate.129,130
Comprehensive examination of these patients is important to rule out other entities that may present with edema and granulomatosis, especially Crohn disease and sarcoidosis (Table 3). Recommended investigations include biopsy of the lesion and microbiological studies of specimens as well as a chest radiograph and colonoscopy.
The Causes of Vulvar Edema.
| Vulvar Edema |
| Infectious |
| Candidal vulvovaginitis |
| Cellulitis |
| Viral infections: herpes simplex virus, Epstein-Barr virus, and parvovirus |
| Chancroid |
| Lymphogranuloma venereum |
| Granuloma inguinale |
| Tuberculosis |
| Syphilis |
| Actinomycosis |
| Filariasis |
| Noninfectious |
| Inflammatory |
| Contact dermatitis |
| Hidradenitis suppurativa |
| Crohn disease |
| Sarcoidosis |
| Idiopathic granulomatous vulvitis |
| Edema-like subcutaneous tumors |
| Lipomas |
| Bartholin's Cyst |
| Lymphangioma circumscriptum |
| Angiomyxoma |
| Lymphoma |
| Others |
| Iatrogenic: following vulvar surgery or radiotherapy |
| Related to pregnancy |
| Congenital lymphedema |
| Trauma/hematoma |
| Medical conditions: heart failure, hypoalbuminemia, preeclampsia, angioedema |
Even with a complete investigation, differentiating between Crohn disease and idiopathic vulvar granulomatosis can be difficult and the 2 conditions have been reported to occur in association.128 Some authors have proposed the hypothesis that granulomatous vulvitis may in fact be a precursor or part of the spectrum of Crohn disease, although most patients do not develop the latter even after prolonged follow-up. Granulomatous vulvitis has also been associated with granulomatous cheilitis.130
Intralesional or systemic corticosteroids have been used in the initial treatment of acute flares, and have resulted in improvement. Metronidazole, danazol, clofazimine, and antimalarial agents have been used as maintenance therapy with variable results. The prognosis is unknown, but the disease can cause fibrosis and chronic lymphedema.128
Vasculopathic PatternAphthous UlcersVulvar aphthous ulcers have characteristics similar to the oral form. It is now believed that the frequency of this condition has been underestimated.123 The most common site is the inner aspect of the labia minora. The lesions typically have a well-defined irregular border, a depth of between 1 to 2mm, and perilesional erythema is observed. As in the oral variant, the cause is unknown. Diagnosis is usually based on exclusion (Table 4).
The Causes of Vulvar Ulcers or Erosions.
| Infectious |
| Bacterial |
| Syphilis, chancroid, lymphogranuloma venereum, granuloma inguinale, impetigo |
| Viral |
| Herpes simplex, human immunodeficiency virus, Epstein Barr virus, cytomegalovirus, influenza A |
| Fungal |
| Candida |
| Others |
| Leishmaniasis and amoebiasis |
| Noninfectious |
| Inflammatory |
| Blistering |
| Pemphigus vulgaris |
| Bullous pemphigoid |
| Mucous membrane pemphigoid |
| Hailey-Hailey disease |
| Bullous systemic lupus erythematosus |
| Linear immunoglobulin A disease |
| Nonblistering |
| Erosive lichen planus |
| Lichen sclerosus |
| Crohn disease |
| Idiopathic aphthosis |
| Secondary aphthous ulcers |
| Behçet disease |
| Pyoderma gangrenosum |
| Plasma cell vulvitis (Zoon vulvitis) |
| Vitamin B12 deficiency |
| Iron or folate deficiency |
| Acute contact dermatitis. |
| Fixed eruption |
| Erythema multiforme |
| Toxic epidermal necrolysis |
| Autoimmune progesterone dermatitis |
| Hidradenitis suppurativa |
| Acrodermatitis enteropathica |
| Malignant |
| Basal cell carcinoma, squamous cell carcinoma, vulvar intraepithelial neoplasia, extramammary Paget disease, lymphoma, Langerhans cell histiocytosis |
| Injury Related |
| Physical (excoriations, trauma, factitious) |
| Chemical (antiseptics, fluorouracil, podophyllotoxin) |
A special variant of these lesions are Lipschütz ulcers, first described by the eponymous author in 1913.131 This variant is characterized by the appearance of 1 or more painful ulcers in the context of a syndrome of fever and general malaise. It most often affects young women (particularly virgins) with no prior history. The ulcers, which usually appear on the medial aspect of the labia minora, may adopt a mirrored distribution or kissing pattern. They usually have well-defined raised borders with associated edema and erythema, and a pseudomembranous exudate or a brownish adherent eschar (Fig. 15).132 The etiology of the condition is unknown, but it has been associated with various primary infections. The Epstein-Barr virus133,134 is currently the agent most often implicated, although the disorder has been linked to other viruses, including the influenza A virus.135 The differential diagnosis should include all disorders that present with acute vulvar ulcers. The process is self-limiting and resolves spontaneously,133 although symptomatic treatment is recommended; topical and oral corticosteroids have proven useful.132
Behçet DiseaseBehçet disease is a multisystem chronic inflammatory disease of unknown etiology, characterized by recurring crops of oral and genital aphthous ulcers, ocular lesions, and skin lesions such as erythema nodosum. These symptoms are sometimes accompanied by arthritis or gastrointestinal and neurological disorders. The diagnostic criteria include recurring genital ulcers, which occur in between 57% and 93% of pacientes123 and are, together with oral ulcers, the most characteristic symptom of this disease. Lesions may leave scars, which should be looked for in the absence of active lesions.123 The first-line treatment for genital ulcers in women is considered to be colchicine alone or in combination with penicillin G benzathine. Other treatments include thalidomide, dapsone, ciclosporin, azathioprine, and anti-tumor necrosis factor agents.136
Plasma Cell VulvitisPlasma cell vulvitis was first described by Garnier137 in 1957. Although less common than its male counterpart, this disease can affect the vestibular mucosa, the labia minora, and the periurethral epithelium. The cause is unknown, although an origin in the immune system has been suggested. This entity is also known by other names, such as vulvitis plasmacellularis, Zoon vulvitis, and vulvitis circumscripta plasmacellularis. It may be asymptomatic or cause itching, burning, irritation, or dyspareunia. Clinical presentation takes the form of 1 or more well-defined shiny erythematous plaques with a characteristic brownish-orange color; these are occasionally accompanied by mottled punctiform purpura2 (Fig. 16). The distribution of plaques is usually bilateral and symmetrical. Clinically, it should be differentiated from lichen planus, fixed eruption, and vulvar intraepithelial neoplasia. Biopsy is essential because definitive diagnosis is based on histologic findings, which include a dense bandlike inflammatory infiltrate of plasma cells, atrophic epidermis, moderate spongiosis, and diamond-shaped keratinocytes.23 As the presence of plasma cells is a common finding in the vulvar mucosa, the percentage of these cells must exceed 50% to establish a diagnosis. If the percentage is between 25% and 50% other criteria must be satisfied, such as the presence of hemosiderin deposition and epithelial atrophy.138 No treatment is necessary in asymptomatic patients. Potent topical corticosteroids are the first-line treatment.139 Other treatments include intralesional triamcinolone acetonide, imiquimod,140,141 and topical ciclosporin.142 Topical tacrolimus is not as effective in plasma cell vulvitis as it is in balanitis.143
ConclusionVulvar skin disease is currently a common reason for consulting a dermatologist. These disorders have a significant impact on patients’ quality of life because, apart from their considerable repercussions on sexual relations and intimacy, they are also associated with a high degree of morbidity, anxiety, social opprobrium, as well as fear of malignancy and sexually transmitted disease.
The vulva, like other areas of the skin, can be affected by many diseases of different etiologies, but the particular anatomical and physiologic conditions in this area give rise to certain peculiarities that may make their management more difficult. The clinical pattern of each one of these vulvar dermatoses is varied and, moreover, many of them have almost identical signs and symptoms. Thus, the first step when dealing with this group of skin disease is to obtain a thorough medical history. This can only be achieved with a good doctor-patient relationship, a complete physical examination, and a correct correlation of clinical and histologic findings in the context of a broad multidisciplinary approach in which the dermatologist plays a fundamental role.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Barchino-Ortiz L, et al. Dermatosis inflamatorias vulvares. Actas Dermosifiliogr.2012;103:260-275.