Malignant syphilis is an uncommon form of secondary syphilis associated with HIV infection. Clinically, it is characterized by necrotic nodules and generalized ulcerated lesions. We present 4 cases of malignant syphilis diagnosed after evaluating syphilis cases diagnosed at our hospital between 2012 and 2016. We describe the epidemiologic, clinical, histiopathologic, and serologic characteristics of malignant syphilis and explore its response to treatment and association with HIV infection. Although malignant syphilis is uncommon, there has been an increase in the number of cases published in recent years, particularly in young HIV-positive patients. Malignant syphilis must be contemplated in the differential diagnosis of HIV patients with ulcerated, necrotic lesions.
La sífilis maligna es una forma infrecuente de sífilis secundaria asociada a la infección por el VIH, caracterizada clínicamente por nódulos necróticos y lesiones ulceradas generalizadas. Presentamos 4 pacientes diagnosticados de sífilis maligna tras revisar los casos de sífilis diagnosticados en nuestro centro entre 2012 y 2016. Describimos los aspectos epidemiológicos, clínicos, histopatológicos y serológicos, así como su relación con el VIH y la respuesta al tratamiento. Aunque se trate de una forma de sífilis poco frecuente, en los últimos años ha aumentado el número de casos publicados, principalmente pacientes jóvenes infectados por el VIH. Es necesario incluir la sífilis maligna en el diagnóstico diferencial de pacientes infectados por el VIH con lesiones ulceradas y necróticas.
Malignant syphilis (MS) is an uncommon form of secondary syphilis that presents with ulcerated and necrotic skin lesions. The last 2 decades have seen an increase in the number of published cases, mainly in patients with human immunodeficiency virus (HIV).1–4 Literature on MS is scarce, and is limited mainly to isolated case reports. It is therefore difficult to determine its frequency, pathogenesis, and clinical spectrum. Here we describe the clinical and histological characteristics and treatment response of MS cases diagnosed in our hospital.
Material and MethodsWe performed a retrospective observational study of patients diagnosed with syphilis in our hospital during the period 2012 to 2016. We selected patients who had ulcerated, necrotic skin lesions compatible with MS and fulfilled the criteria of Fisher et al5 for the diagnosis of MS. The clinical histories of participating patients were reviewed and the following variables recorded: sex, age, sexual orientation, mucocutaneous lesions, extracutaneous manifestations, HIV status, viral load and HIV treatment, CD4+ T-lymphocyte count, histological pattern, venereal disease research laboratory (VDRL) test titer at diagnosis, treatment administered, and treatment response.
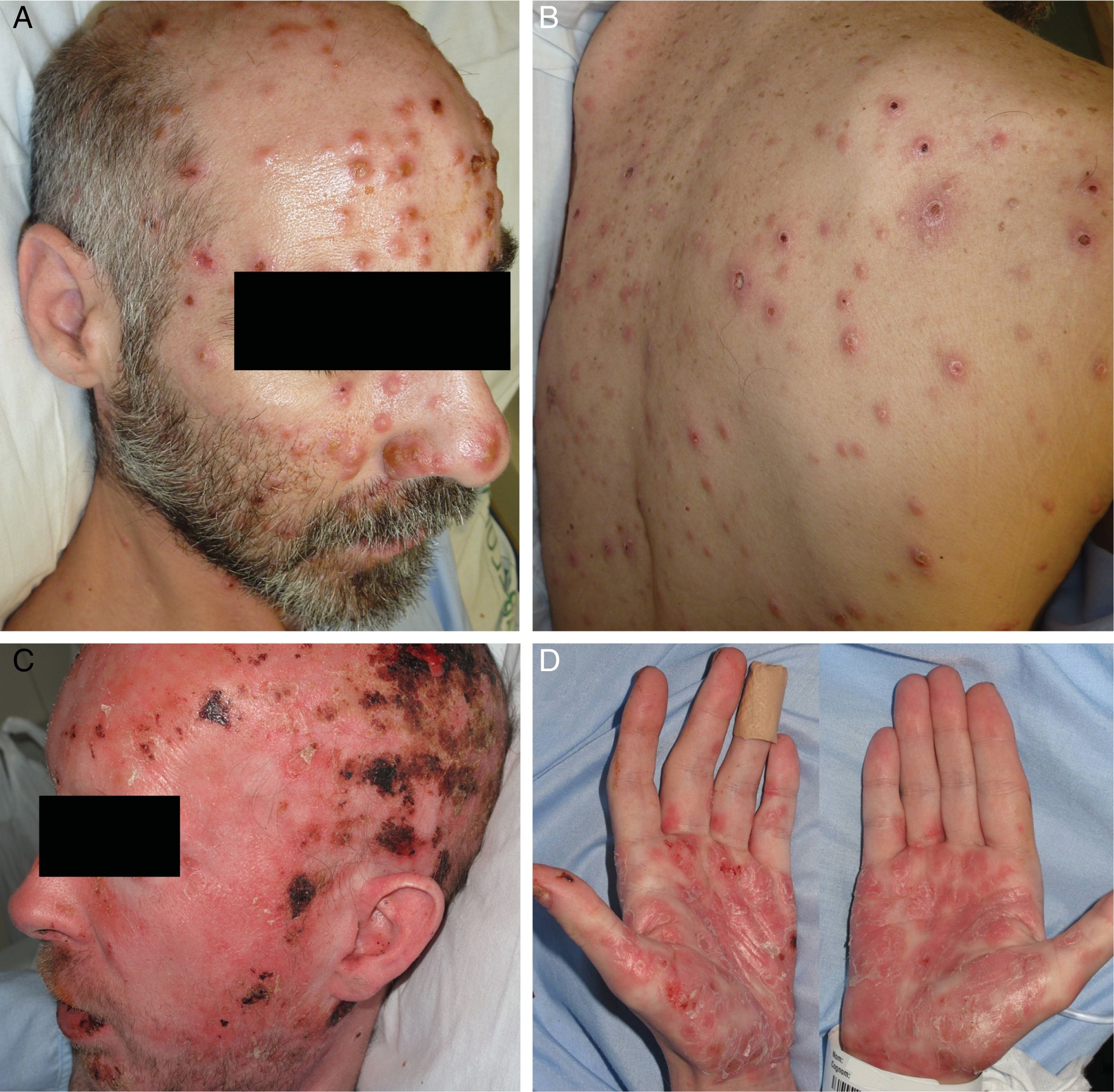
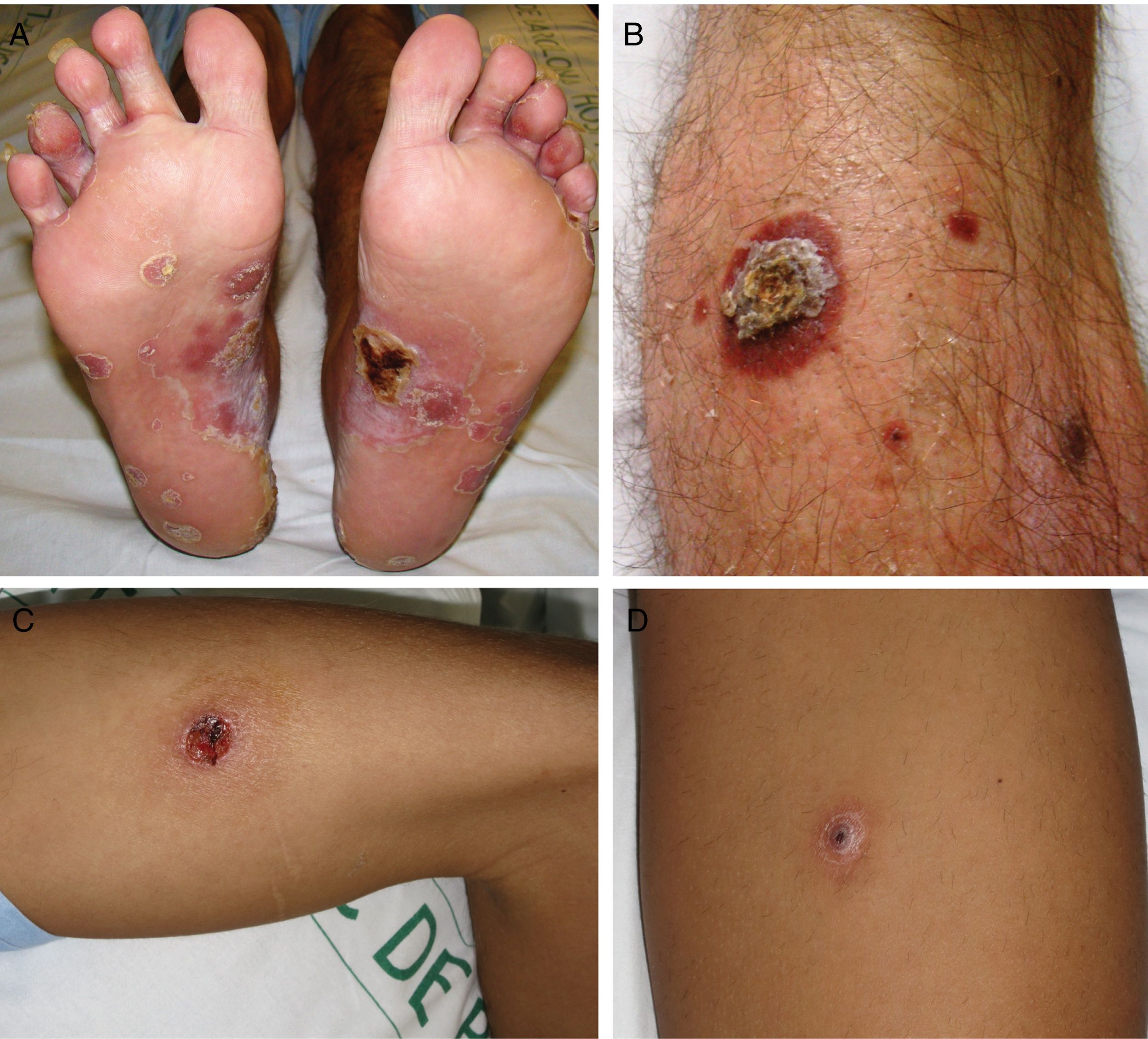
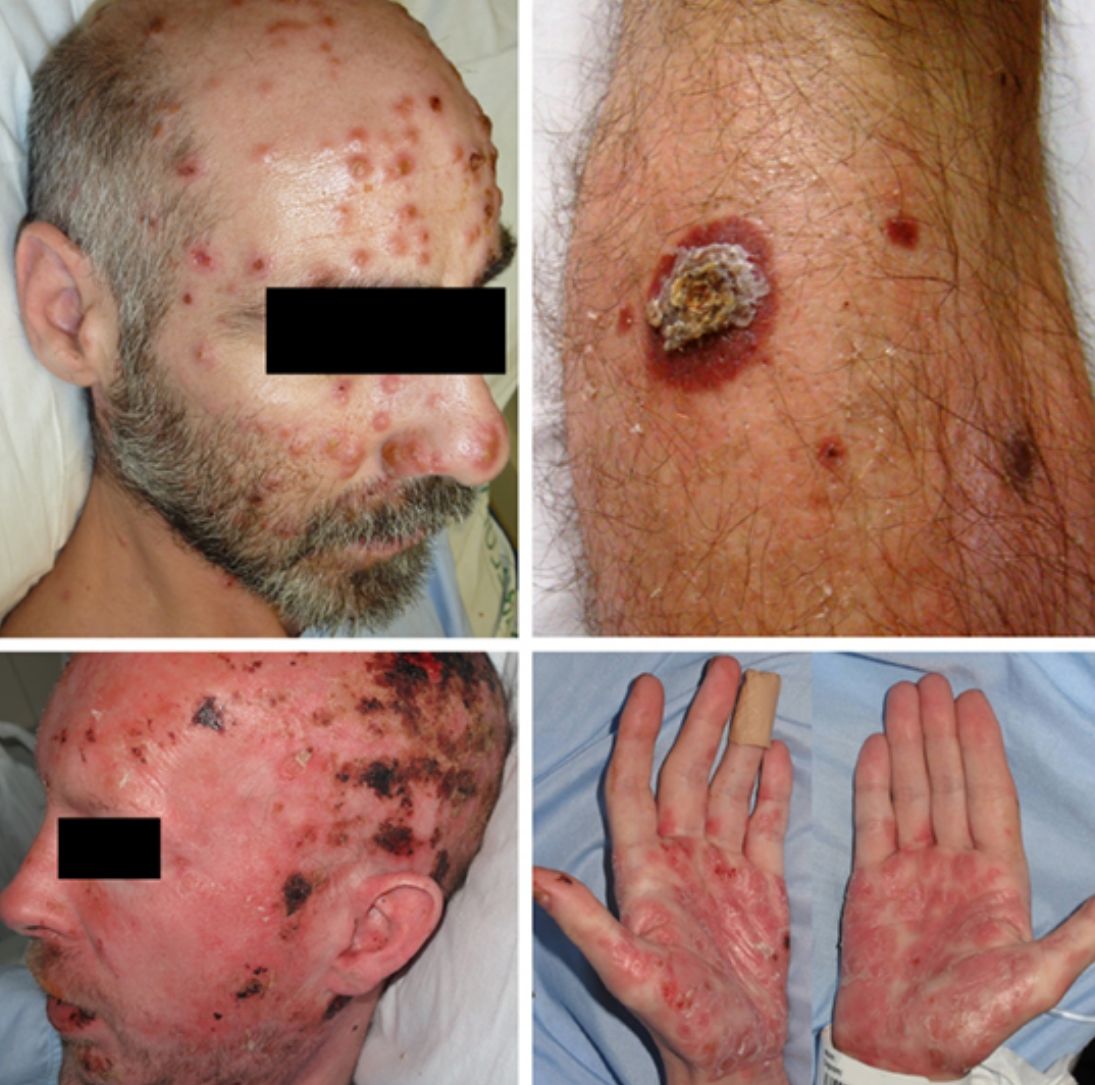
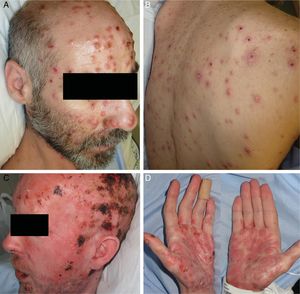
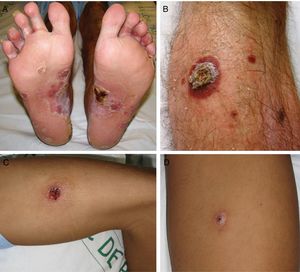
ResultsA total of 332 patients were diagnosed with syphilis during the study period, of whom 202 were HIV-positive. Four cases corresponded to MS, accounting for 1.2% of the total number of diagnosed syphilis cases and 2% of syphilis cases in HIV-positive patients. The affected individuals were men who had sex with other men, were positive for HIV, and aged between 26 and 54 years (Table 1). All had multiple ulcerated and necrotic nodules with palmoplantar involvement, and scalp involvement was observed in 3 cases (Figs. 1 and 2). A clinical diagnosis of neurosyphilis was established in one case, and was confirmed by cerebrospinal fluid analysis.
Clinical and Histological Characteristics, Blood Test Abnormalities, Treatment, and Clinical Course
| Patient | Sex | Age, y | Mucocutaneous Signs | Systemic Signs | Days Since Lesion Onset | HIV Status | HIV Viral Load, Copies/mL | CD4+ Count, Cells/mm3 | Histological Pattern (Cellularity of Dermal Inflammatory Infiltrate) | VDRL Titer | Treatment | Clinical Course |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M (MSM) | 39 | Ulcerated and necrotic nodules, palmoplantar and scalp involvement | Fever (>38°C) | 14 | + (not in treatment) | 24 950 | 171 | Lichenoid dermatitis (lymphocytes, histiocytes, plasma cells). Treponema immunostaining + | 1:128 (prozone phenomenon) | Intramuscular penicillin G benzathine, 2.4 MU (3 doses) | Jarisch-Herxheimer reaction. Complete clinical response |
| 2 | M (MSM) | 36 | Ulcerated and necrotic nodules, palmoplantar, ungueal, and scalp involvement. Oral erosions. Genital involvement, seborrheic dermatitis-like lesions. Perianal condyloma lata | Fever (>38°C) Syphilitic uveitis. Neurosyphilis | 21 | + (not in treatment) | 1 350 000 | 250 | Spongiotic dermatitis (histiocytes, plasma cells). Treponema immunostaining + | 1:250 (prozone phenomenon) | Intravenous aqueous penicillin G, 24 MU (daily for 15 days) | Jarisch-Herxheimer reaction. Complete clinical response |
| 3 | M (MSM) | 54 | Ulcerated and necrotic nodules, hyperkeratotic nodules, palmoplantar involvement | Fever (>38°C) | 30 | + (unknown, not in treatment) | 71 300 | 697 | Lichenoid dermatitis (lymphocytes, histiocytes, plasma cells). Treponema immunostaining - | 1:512 | Intramuscular penicillin G benzathine, 2.4 MU (3 doses) | Complete clinical response |
| 4 | M (MSM) | 26 | Ulcerated and necrotic nodules, palmar and scalp involvement | Low-grade fever, night sweats | 30 | + (in treatment) | <37 | 790 | Cutaneous abscess (lymphocytes, histiocytes, plasma cells). Treponema immunostaining - | 1:256 | Intramuscular penicillin G benzathine, 2.4 MU (1 dose) | Complete clinical response |
Abbreviations: HIV, human immunodeficiency virus; M, male; MSM, men who have sex with men; MU, millions of units; VDRL, venereal disease research laboratory test.
Three of the 4 MS patients were not receiving HIV treatment at the moment of syphilis diagnosis, and one was unaware of his HIV-positive status. All patients had positive results in treponemal and nontreponemal tests, and a prozone phenomenon was observed in the 2 patients with the lowest CD4+ counts. Both these patients also displayed a Jarisch-Herxheimer reaction after antibiotic treatment.
All patients had achieved complete clinical remission at the follow-up visit 1 month after treatment. A 4-fold decrease in VDRL titer was observed after 3 months in 2 patients and after 6 months in 1 patient. The fourth patient did not attend the scheduled follow-up visits. After 1 year of follow-up, none of the patients in follow-up had developed new lesions or showed increases in VDRL titer.
DiscussionMS is an uncommon form of secondary syphilis, the exact incidence of which is unknown.1,2 The first case of MS in a HIV patient was reported in in 1988.6 Since then, there has been an increase in the number of cases of MS in young, HIV-positive men.1,7 Immunological status does not appear to influence the development of MS; 80% of HIV patients with MS have a CD4+ cell count >200 cells/mm3 and almost none have had a previous opportunistic infection.2,8 These observations suggest that MS is the consequence of either an interaction between Treponema pallidum and the HIV virus or a functional immunological defect, rather than the result of a quantitative immunological deficit.4,9 In our series, only one patient had a CD4+ cell count <200 cells/mm3 at the time of diagnosis of syphilis, and CD4+ counts >500 cells/mm3 were detected in 2 patients, one of whom was receiving appropriate antiretroviral treatment and had an undetectable viral load. Cases of MS have also been described in nonimmunocompromised, HIV-negative individuals.7,10
Clinically, MS is characterized by disseminated pustules that evolve to ulcerated nodules, with a necrotic or hyperkeratotic surface, occasionally with a rupioid or ostraceous appearance.1,3 The lesions mainly affect the trunk and extremities, but involvement of the mucosae, palms, soles, and scalp is also common.4 Fever and constitutional symptoms are frequent and often precede cutaneous signs.11 The aggressiveness classically attributed to this form of syphilis is evidenced by the appearance of necrotic lesions that often leave varioliform scars after healing. These lesions, together with a high fever and general malaise, seem to be the only consistent features of MS that account for its more aggressive course compared with other forms of secondary syphilis. The extracutaneous involvement reported in these cases (neurosyphilis,12 hepatitis,11 and ocular involvement7,13) has also been described in other forms of secondary syphilis, and there is no evidence in the literature to indicate that it is more frequent in MS.
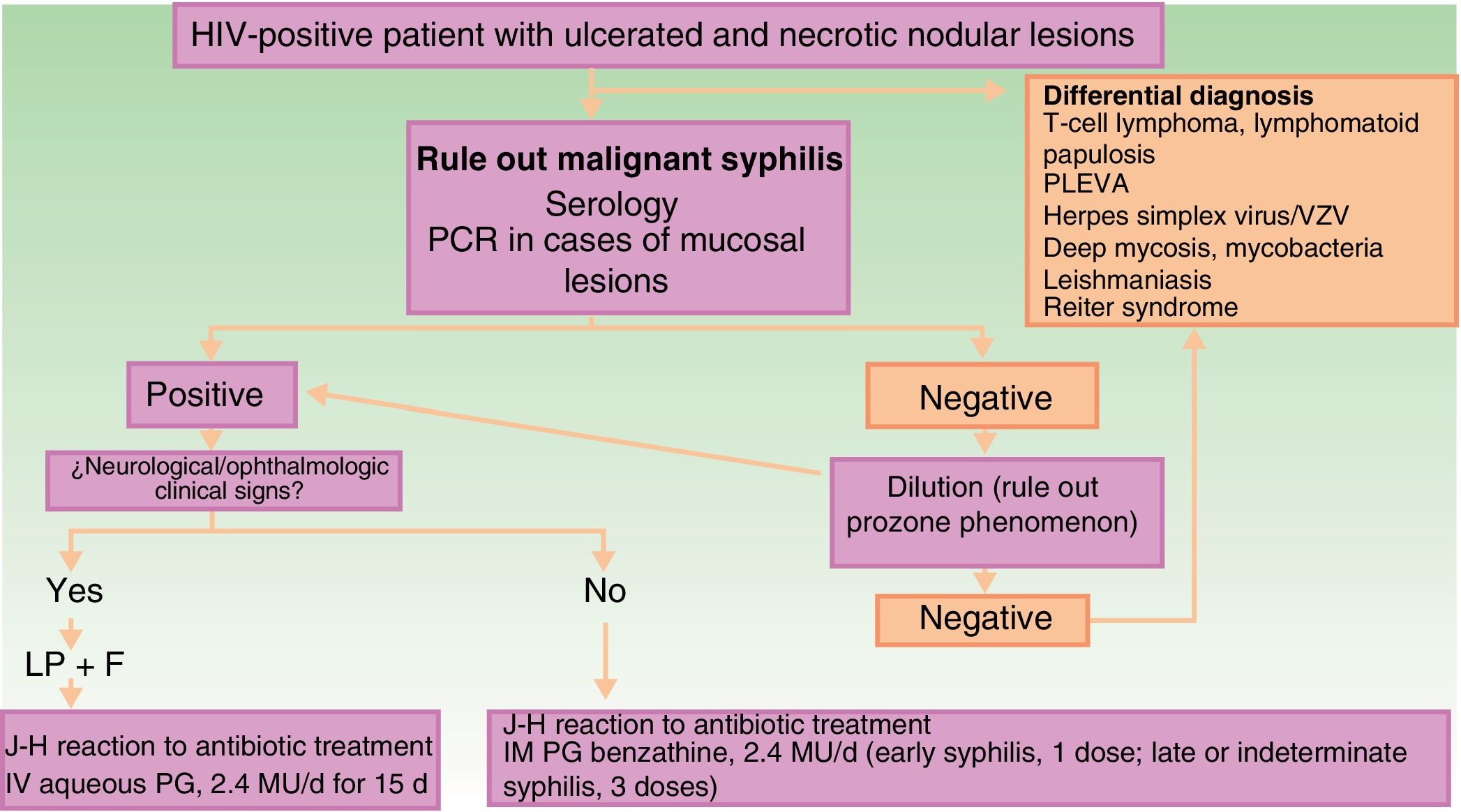
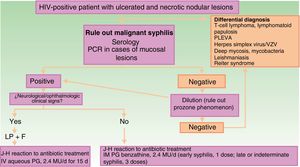
The clinical differential diagnosis can be challenging, and should include other infectious skin diseases (mainly infections caused by herpes family viruses, ecthyma gangrenosum, deep mycoses, mycobacteriosis, and leishmaniasis), lymphoproliferative skin diseases (cutaneous T-cell lymphoma, lymphomatoid papulosis, and pityriasis lichenoides et varioliformis acuta), and other diseases such as Reiter syndrome (Fig. 3).
Schematic showing diagnostic and treatment approach for suspected malignant syphilis. Abbreviations: F, fundus; HIV, human immunodeficiency virus; IHC, immunohistochemistry; IM, intramuscular; IV, intravenous; J-H, Jarisch-Herxheimer; LP, lumbar puncture; MU, millions of units; PCR, polymerase chain reaction; PG, penicillin G; PLEVA, pityriasis lichenoides et varioliformis acuta; VZV, varicella-zoster virus.
Fisher et al5 defined the classical diagnostic criteria for MS: compatible macroscopic and microscopic skin lesions; a high VDRL titer; Jarisch-Herxheimer reaction upon starting antibiotic treatment; and rapid clinical resolution with treatment. All the cases in our series fulfilled these criteria, although 2 patients did not develop a Jarisch-Herxheimer reaction in response to antibiotic treatment. The absence of this reaction was not considered a reason to rule out a diagnosis of MS, nor does it appear to have any bearing on the specific clinical presentation. Indeed, the absence of the Jarisch-Herxheimer reaction has been described in many MS cases, and its presence may be difficult to confirm in patients who are already febrile and in a poor general condition as a consequence of the disease.
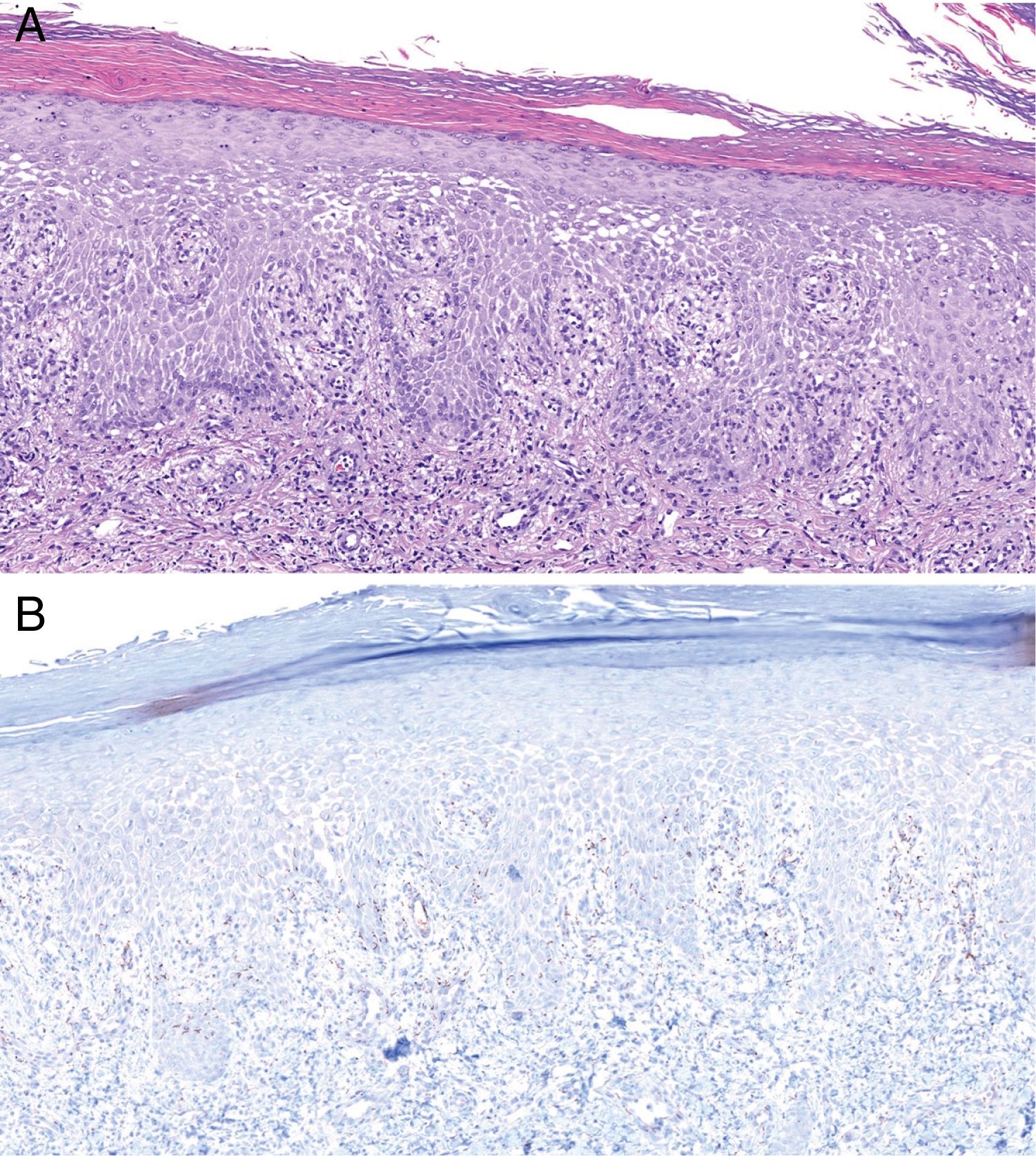
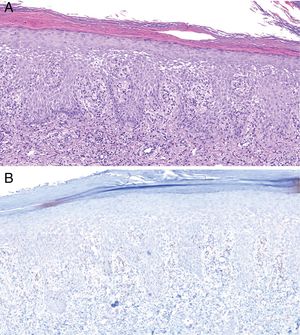
Histologically, MS is similar to other forms of secondary syphilis, and is characterized by the presence of a dense inflammatory infiltrate, sometimes in the context of a lichenoid dermatitis pattern, with a predominance of lymphocytes and plasma cells and occasional granulomas.4 Ulcerated lesions may be caused by vascular involvement secondary to infection, which gives rise to infarcts in medium-sized arteries.8 In our series, we observed vascular thrombosis (without signs of vasculitis) in only one case, probably because these findings depend on the lesion biopsied and its stage of evolution. Classically, T pallidum is absent from MS histology (probably due to the intensity of the inflammatory infiltrate).11 However, in our series we detected its presence by immunohistochemistry in 2 patients (Fig. 4).
Histology of biopsy from Patient 2. A, Epidermis showing marked spongiotic changes. Predominantly histiocytic inflammatory infiltrate is evident in the superficial dermis (hematoxylin-eosin, original magnification ×100). B, Visualization of abundant spirochetes by immunostaining for Treponema pallidum (Treponema immunohistochemistry, original magnification ×100).
There is no evidence in the literature indicating a greater incidence of treatment failure and neurosyphilis in MS as compared with other forms of secondary syphilis in patients with HIV. Although the most frequently reported regimen for the treatment of these cases is 3 doses of intramuscular penicillin benzathine (2.4 million units), treatment with a single dose results in a comparable response and has no effect on the rate of treatment failure.4 In these patients it thus seems appropriate to opt for the therapeutic approach recommended in current clinical guidelines14 for the management of HIV-positive patients with syphilis. Therefore, lumbar puncture should always be performed in MS patients with signs or symptoms suggestive of neurosyphilis or ocular involvement. Lumbar puncture is also recommended in HIV patients with late or unconfirmed syphilis, with a CD4+ count ≤350 cells/mm3 and/or persistent VDRL/rapid plasma reagin (RPR) titers >1:32 after treatment, in the absence of neurological signs.14
ConclusionMS is an uncommon variant of secondary syphilis that should be included in the differential diagnosis of HIV patients with ulcerated and necrotic lesions. In our series, MS accounted for 1.2% of all patients diagnosed with syphilis. MS is characterized not by aggressive systemic manifestations but by aggressive cutaneous lesions that heal leaving varioliform scars. In the absence of neurosyphilis, the proposed treatment is the same as that recommended for HIV-negative patients with secondary syphilis. A single dose of intramuscular penicillin is sufficient in cases of early syphilis.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Irene Fuertes-de Vega, José-Luís Blanco, Anna González, Asunción Moreno, Miriam Álvarez, Jordi Bosch
Please cite this article as: Fustà-Novell X, Morgado-Carrasco D, Barreiro-Capurro A, Manzardo C, Alsina-Gibert M, Miembros del Grupo de Trabajo de Infecciones de Transmisión Sexual del Hospital Clínic de Barcelona, et al. Syphilis Maligna: A Presentation to Bear in Mind. Actas Dermosifiliogr. 2019;110:232–237.