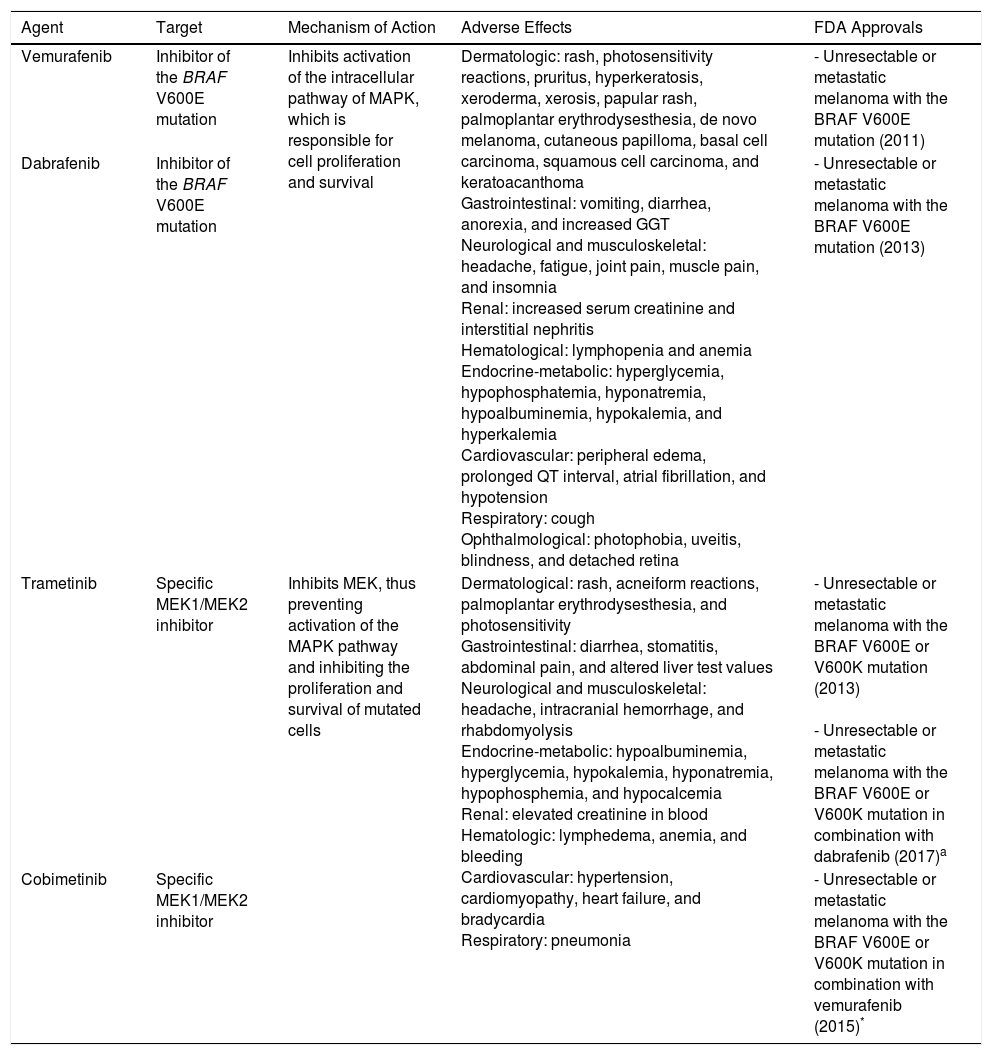
At present, the approved adjuvant drugs for advanced melanoma are interferon, which has very limited activity, and ipilimumab, which is associated with considerable toxicity and up to 1% mortality.1 Targeted therapy (anti-BRAF antibody, anti-MEK antibody, and their combinations) (Table 1) and immunotherapy with anti-PD1 antibodies have modified the prognosis of metastatic melanoma,2 although no sufficient evidence has become available to date on their effectiveness as adjuvant approaches. Long et al.3 recently published the results of a phase III, double-blind clinical trial in patients with stage IIIA-C BRAF-mutated melanoma. The authors compared placebo (n=432) with oral combination targeted therapy (CTT) (n=438) based on dabrafenib (anti-BRAF, 300mg/d) and trametinib (anti-MEK, 2mg/d) for 12 months. At a median follow-up of 2.8 years, CTT had led to a 53% reduction in the risk of relapse compared with placebo. The estimated recurrence-free survival rate (RFSR) at 3 years was 58% in the CTT group compared with 39% in the placebo group (HR, 0.47; 95% CI, 0.39-0.58; P<.001). The estimated overall survival rate was 86% and 77%, respectively (HR, 0.57; 95% CI, 0.42-0.79; P=.0006). Distant metastasis–free survival was also higher with CTT. At the time of the statistical analysis, 60 patients had died in the CTT group (14%) and 93 patients had died in the placebo group (22%). Severe adverse events were recorded in 36% of CTT patients (114 had to interrupt treatment and 1 died of pneumonia) and in 10% of placebo patients.
Anti-BRAF and Anti-MEK Drugs Used in the Treatment of Melanoma.
| Agent | Target | Mechanism of Action | Adverse Effects | FDA Approvals |
|---|---|---|---|---|
| Vemurafenib | Inhibitor of the BRAF V600E mutation | Inhibits activation of the intracellular pathway of MAPK, which is responsible for cell proliferation and survival | Dermatologic: rash, photosensitivity reactions, pruritus, hyperkeratosis, xeroderma, xerosis, papular rash, palmoplantar erythrodysesthesia, de novo melanoma, cutaneous papilloma, basal cell carcinoma, squamous cell carcinoma, and keratoacanthoma Gastrointestinal: vomiting, diarrhea, anorexia, and increased GGT Neurological and musculoskeletal: headache, fatigue, joint pain, muscle pain, and insomnia Renal: increased serum creatinine and interstitial nephritis Hematological: lymphopenia and anemia Endocrine-metabolic: hyperglycemia, hypophosphatemia, hyponatremia, hypoalbuminemia, hypokalemia, and hyperkalemia Cardiovascular: peripheral edema, prolonged QT interval, atrial fibrillation, and hypotension Respiratory: cough Ophthalmological: photophobia, uveitis, blindness, and detached retina | - Unresectable or metastatic melanoma with the BRAF V600E mutation (2011) |
| Dabrafenib | Inhibitor of the BRAF V600E mutation | - Unresectable or metastatic melanoma with the BRAF V600E mutation (2013) | ||
| Trametinib | Specific MEK1/MEK2 inhibitor | Inhibits MEK, thus preventing activation of the MAPK pathway and inhibiting the proliferation and survival of mutated cells | Dermatological: rash, acneiform reactions, palmoplantar erythrodysesthesia, and photosensitivity Gastrointestinal: diarrhea, stomatitis, abdominal pain, and altered liver test values Neurological and musculoskeletal: headache, intracranial hemorrhage, and rhabdomyolysis Endocrine-metabolic: hypoalbuminemia, hyperglycemia, hypokalemia, hyponatremia, hypophosphemia, and hypocalcemia Renal: elevated creatinine in blood Hematologic: lymphedema, anemia, and bleeding Cardiovascular: hypertension, cardiomyopathy, heart failure, and bradycardia Respiratory: pneumonia | - Unresectable or metastatic melanoma with the BRAF V600E or V600K mutation (2013) - Unresectable or metastatic melanoma with the BRAF V600E or V600K mutation in combination with dabrafenib (2017)a |
| Cobimetinib | Specific MEK1/MEK2 inhibitor | - Unresectable or metastatic melanoma with the BRAF V600E or V600K mutation in combination with vemurafenib (2015)* |
Source: Luke et al.2 and Carlos et al..4
Abbreviations
FDA, United States Food and Drug Administration; GGT, γ-glutamyl transferase; MAPK, mitogen-activated protein kinase; MEK, MAPK extracellular signal-regulated kinase.
aCombination target therapy with anti-BRAF and anti-MEK (vemurafenib-cobimetinib, dabrafenib-trametinib) provides a better response to therapy and significantly fewer adverse effects than anti-BRAF or anti-MEK agents in monotherapy.2,4
Weber et al.5 reported the results of a randomized, double-blind, phase III clinical trial in patients with disease-free stage IIIB-C and IV melanoma in which they compared the anti-PD1 agent nivolumab (n=453) (3mg/kg/2 wk) with ipilimumab (n=453) (10mg/kg/3 wk, 4 doses then every 12 weeks). Both treatments were administered for 12 months with a minimum follow-up of 18 months. The RFSR at 12 months was 70.5% with nivolumab and 60.8% with ipilimumab (HR, 0.65; 97.56% CI, 0.51-0.83; P<.001). Nivolumab was seen to be superior to ipilimumab in almost all of the subgroups analyzed, including those with expression of PD-1<5% (RFSR, 64.3% and 53.7%, respectively), stages IIIB-C (RFSR, 72.3% and 61.6%, respectively), ulcerated tumors, macroscopic and microscopic nodal disease, and tumors with(out) mutations in BRAF. The severe adverse effect rate (grades 3 and 4) was 14.4% with nivolumab and 45.9% with ipilimumab. Two patients died as a result of treatment with ipilimumab (marrow aplasia and colitis).5
The results of these clinical trials show a significant increase in disease-free survival in patients with advanced melanoma treated with CTT or nivolumab. The toxicity associated with these drugs could be considered acceptable if the results are eventually reproducible in the long term.
We are living in an age of enormous changes in the treatment of melanoma. Dermatologists must become familiar with these new drugs, their indications, and their adverse effects.
Please cite this article as: Morgado-Carrasco D, Terc F, Ertekin SS, Ferrandiz L. FR-Novedades en la terapia adyuvante del melanoma cutáneo avanzado. Actas Dermosifiliogr. 2019;110:238–240.