Atopic dermatitis (AD) is a chronic inflammatory skin disease that typically affects children. Severe forms may have a profound effect on patients’ quality of life. Some forms are resistant to conventional treatment and require the use of systemic immunosuppressants such as azathioprine (AZA) to adequately manage the disease.
ObjectiveTo evaluate the effectiveness and tolerance of AZA in children with severe AD.
Patients and methodsWe performed a retrospective study of children with severe AD treated with AZA between January 2007 and May 2017.
ResultsWe reviewed the cases of 11 patients (6 boys and 5 girls) with a mean age of 13 years (range, 8-18 years). The mean (SD) age at start of treatment was 10.9 (2.2) years (95% CI 8.6-13.1). The mean initial dosage of AZA was 1.8 (0.2) mg/kg/d. We evaluated treatment response after 4 weeks, 12 to 16 weeks, and 6 months. Mean treatment duration was 10.8 (5.7) months. Treatment had to be suspended in 2 patients because of adverse effects. Seven of the 9 remaining patients presented complete or almost complete clearance of the AD after 6 months of treatment.
ConclusionIn our experience, AZA is well tolerated and may be considered as a treatment option in children with severe AD resistant to conventional treatment.
La dermatitis atópica (DA) es una enfermedad inflamatoria crónica de la piel típicamente infantil cuyas formas graves pueden afectar intensamente la calidad de vida del paciente. Existen formas refractarias al tratamiento convencional en las que es preciso emplear inmunosupresores sistémicos como la azatioprina (AZA) para alcanzar un buen control de la enfermedad.
ObjetivoEvaluar la eficacia y la tolerancia de la AZA en niños con DA grave.
Pacientes y métodosSe realizó una revisión retrospectiva de niños con DA grave tratados con AZA entre enero de 2007 y mayo de 2017.
ResultadosSe revisaron 11 pacientes (6 varones, 5 mujeres) con una edad promedio de 13 años (rango 8-18 años). La edad media±DE al inicio del tratamiento fue de 10,9±2,2 años (IC 95% 8,6-13,1). La media de la dosis inicial de AZA fue de 1,8±0,2mg/kg/d. Evaluamos la respuesta al tratamiento de nuestros pacientes a las 4 semanas, entre la semana 12 y la 16, y a partir de los 6 meses. La media del tratamiento fue de 10,8±5,7 meses. Dos pacientes tuvieron que suspender el tratamiento por efectos adversos. Siete de los 9 pacientes restantes presentaron un aclaramiento completo o casi completo de la DA a los 6 meses de tratamiento.
ConclusiónEn nuestra experiencia, la AZA es bien tolerada y puede ser considerada como una opción terapéutica en los niños con DA grave refractaria a tratamientos convencionales.
Atopic dermatitis is a chronic inflammatory disease of childhood that is characterized by recurrent outbreaks of very pruriginous lesions. Some children have severe disease that is refractory to conventional treatment with topical corticosteroids, thus necessitating immunosuppressive therapy to achieve control.1 Azathioprine is a prodrug that is converted into 6-mercaptopurine after absorption in the intestine. 6-Mercaptopurine is subsequently metabolized in the liver and gastrointestinal tract by 3 main enzymes: hypoxanthine-guanine phosphoribosyltransferase, xanthine oxidase, and thiopurine methyltransferase. Azathioprine antagonizes purine metabolism and thus inhibits the synthesis of DNA, RNA, and proteins, as well as cell mitosis.2 It is used in dermatology as a corticosteroid-sparing agent in a wide variety of inflammatory and autoimmune diseases, including (off-label) atopic dermatitis and nummular eczema.3–5 Its most common adverse effects are myelosuppression, liver toxicity, gastrointestinal symptoms, hypersensitivity reactions, and pancreatitis. Measurement of the activity of the enzyme thiopurine methyltransferase in serum, which varies depending on specific gene polymorphisms,1 helps to prevent some of these adverse effects, mainly myelosuppression. Given that the agent is not authorized for infantile atopic dermatitis, it is reserved for specific cases where topical treatments or other types of systemic treatment are not effective.
ObjectivesTo evaluate the efficacy and tolerance of azathioprine in children with severe atopic dermatitis that is refractory to conventional approaches.
Material and methodsWe performed a single-center retrospective observational cohort study of 11 patients with severe atopic dermatitis treated with azathioprine at Hospital Infantil Universitario Niño Jesús, Madrid, Spain between January 2007 and May 2017. The local ethics committee approved the study. Patients were identified using computerized and imaging databases. Atopic dermatitis was defined using the Investigator Global Assessment scale, which defined the disease as the presence of intense, dark erythema with induration, excoriated papules, exudation, and crusting.6,7 Before receiving azathioprine, all patients had had a poor response to standard treatment with moderate-potency topical corticosteroids (class 3) applied twice daily or once daily with wet-wrap dressing techniques and/or topical immunomodulators. No patients received oral azathioprine as their first line of immunosuppressive therapy: 10 had previously received ciclosporin A, and 1 had received methotrexate. Patients were allowed to apply moderate-potency topical corticosteroids during treatment with azathioprine. In all cases, levels of thiopurine methyltransferase were assessed before starting treatment, and the dose of azathioprine was adjusted according to outcome (< 5.1U/mL red cells, not administered; 5.1-13.7U/mL, 0.5mg/kg/d; 13.8-18.0U/mL, 1.5mg/kg/d; 18.1-26.0U/mL, 2.5mg/kg/d; and 26.1-40U/mL, 3.0mg/kg/d).
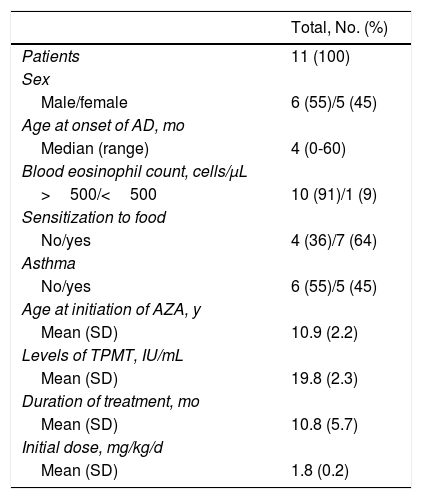
The demographic data recorded included sex, age at onset of atopic dermatitis, age at initiation of treatment, history of asthma and/or sensitization to food (defined as specific immunoglobulin [Ig] E levels for food greater than 95% of the positive predictive value), total IgE levels, and eosinophilia in blood (defined as peripheral eosinophilia> 500/μL). Drug-related variables included the initial dose of azathioprine, dose increases (yes/no), and duration of treatment. Outcome was assessed based on the clinical response after 4 weeks of treatment, after 3-4 months of treatment, and at>6 months of treatment and on long-term efficacy (time to relapse and disease-free period after withdrawal of azathioprine). Given that the Investigator Global Assessment scale is difficult to evaluate in a retrospective study, evaluation was based on the clinical notes of the attending dermatologist, with outcome classified as complete or near-complete clearance (improvement≥90%), marked improvement (50%-89%), slight improvement (<50%), and failure (no improvement). In the case of good to excellent outcome (complete clearance, near-complete clearance, or marked clearance), the disease was considered to be in remission when the patient's condition had resolved and could be managed with topical therapy (moderate-potency corticosteroids/topical immunomodulators) and to be recurrent when flares made it advisable to administer another systemic immunosuppressive drug.
Laboratory monitoring consisted of a complete blood count and biochemistry before initiation of treatment, 15 days after initiation, 4 weeks after initiation, and every 3 months thereafter. Clinical follow-up was at least at the same intervals. The assessment of adverse effects included symptoms (eg, asthenia), gastrointestinal adverse effects (eg, nausea, vomiting, and abdominal pain), physical examination, complete blood count, serum urea, creatinine, and serum liver enzymes. Any complaint that could be considered a clinical adverse effect of azathioprine was also reported.
Statistical analysisData are expressed as mean (SD) or median (range) in the case of nonnormally distributed values. Differences between means were assessed using the t test or Mann-Whitney test in the case of nonnormally distributed variables. The χ2 test or Fisher exact test was used to determine the association between qualitative variables. All of the analyses were performed using Wizard for Mac, version 1.9.9.
ResultsWe reviewed 11 patients (6 boys, 5 girls) with a mean age of 13 years (range, 8-18 years). Mean age at initiation of treatment was 10.9 (2.2) years (95% CI, 8.6-13.1 years). The mean initial dose was 1.8 (0.2) mg/kg/d. We evaluated the response to treatment at 4 weeks, between weeks 12 and 16, and from 6 months onward. One patient remains in follow-up.
Table 1 shows demographic data and drug-related variables. Two patients had to suspend therapy with azathioprine because of adverse effects, one during the first month and the other at the fourth month after starting treatment. In the group of patients who completed treatment with azathioprine, the mean duration of therapy was 10.8 (5.7) months (95% CI, 5.0-16.6). After 1 month of treatment, no patients had a complete or near-complete response. Between 12 and 16 weeks, clearance was near-complete in 4 of 9 patients, a marked improvement was observed in 3 patients, and a slight improvement in 2. Two of the 3 patients who had improved at the fourth month continued to improve until near-complete clearance was observed at the sixth month of treatment. Therefore, of the 9 patients whose treatment was extended for a minimum of 6 months, clearance was almost complete in 6 at this point.
Demographic Data and Drug-Related Variables.
| Total, No. (%) | |
|---|---|
| Patients | 11 (100) |
| Sex | |
| Male/female | 6 (55)/5 (45) |
| Age at onset of AD, mo | |
| Median (range) | 4 (0-60) |
| Blood eosinophil count, cells/μL | |
| >500/<500 | 10 (91)/1 (9) |
| Sensitization to food | |
| No/yes | 4 (36)/7 (64) |
| Asthma | |
| No/yes | 6 (55)/5 (45) |
| Age at initiation of AZA, y | |
| Mean (SD) | 10.9 (2.2) |
| Levels of TPMT, IU/mL | |
| Mean (SD) | 19.8 (2.3) |
| Duration of treatment, mo | |
| Mean (SD) | 10.8 (5.7) |
| Initial dose, mg/kg/d | |
| Mean (SD) | 1.8 (0.2) |
Abbreviations: AD, atopic dermatitis; AZA, azathioprine; TPMT, thiopurine methyltransferase.
We found no statistically significant association between response to treatment and a history of allergic sensitization, history of asthma, eosinophilia, and IgE levels.
The mean dose of azathioprine used in the present study was 1.8 (0.2) mg/kg/d (95% CI, 1.5-2.1). The mean follow-up time was 33.1 (12.0) months. One patient was still taking azathioprine at the end of the study. After suspension of treatment, 2 patients with complete clearance required new systemic therapy at 8 and 20 months, respectively. The remaining cases were managed with topical corticosteroids, immunomodulators, or both.
Adverse effects were recorded in 3 patients (27%). One patient experienced nausea and epigastric pain, one experienced a mild increase in transaminases, and a third patient experienced a mild transitory increase in transaminases that resolved spontaneously. In the case of the 2 patients with epigastric pain, treatment was suspended owing to the intensity of the pain. No patients had a severe intercurrent infection or complained of effects other than those already commented upon.
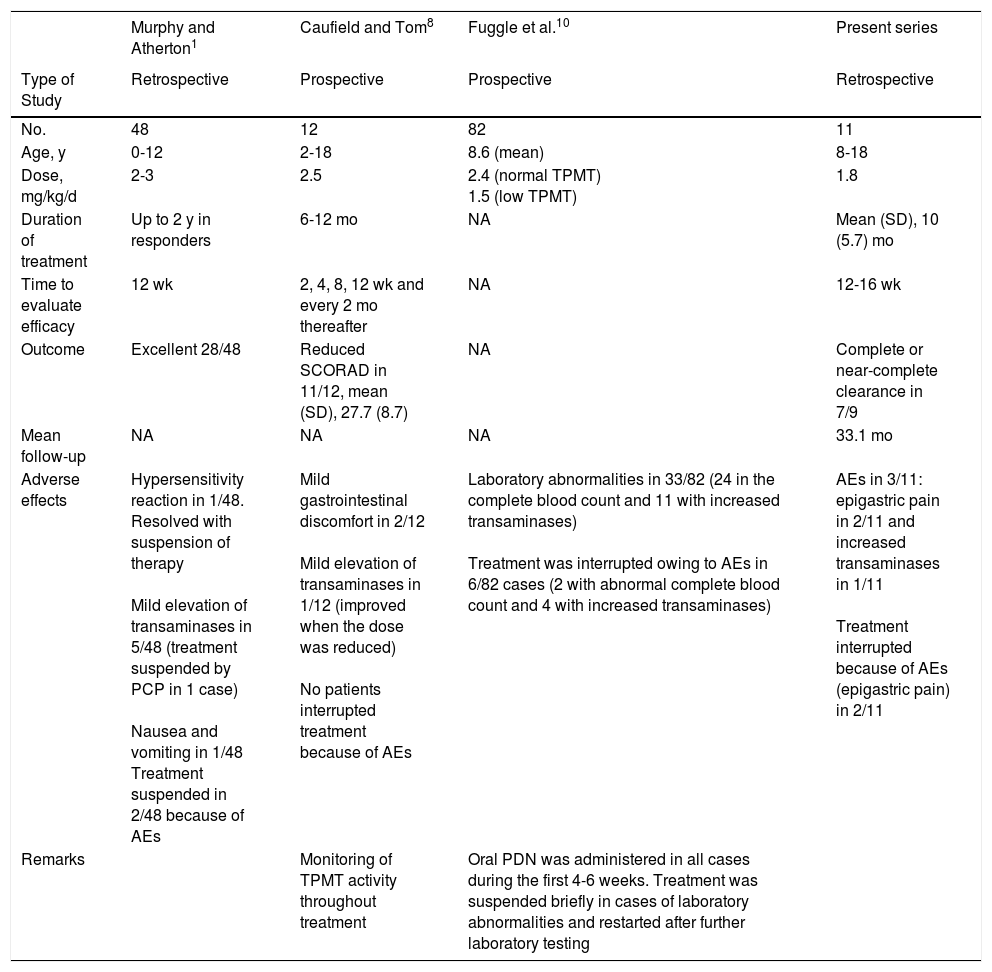
DiscussionAlthough azathioprine is not authorized by the United Stated Food and Drug Administration or the European Medicines Agency for the treatment of atopic dermatitis in children, it has proven beneficial in 2 randomized placebo-controlled trials in adults with atopic dermatitis treated with azathioprine for 12 weeks and in several retrospective pediatric case series.4,8–10 Both our results and those of previous studies1,10 that evaluated the efficacy and tolerability of azathioprine in children with severe atopic dermatitis indicate that most patients have a good or very good response at 12-16 weeks after starting treatment. Thus, in our study, clearance was complete or near-complete in 7 of 9 patients; this figure is slightly higher than that reported elsewhere.9Table 2 shows studies that analyzed treatment with azathioprine in pediatric patients with atopic dermatitis. The main disadvantage of azathioprine is not its efficacy, which is similar to that of other immunosuppressants (eg, ciclosporin and methotrexate),11,12 but the time to response, since it does not take effect for at least 3 months.10 In fact, consistent with the view of other European specialists,13,14 this interval and the adverse effects profile meant that azathioprine was not our first choice for any of the patients. We also agree with other authors that the adverse effects we observed were mainly mild gastrointestinal adverse effects, which led to suspension of treatment in only 2 cases.5,10 It is noteworthy that in both cases, these effects appeared during the first month of treatment and resolved once medication was suspended.
Summary of Data From Studies on Atopic Dermatitis Treated With Azathioprine in Children.
| Murphy and Atherton1 | Caufield and Tom8 | Fuggle et al.10 | Present series | |
|---|---|---|---|---|
| Type of Study | Retrospective | Prospective | Prospective | Retrospective |
| No. | 48 | 12 | 82 | 11 |
| Age, y | 0-12 | 2-18 | 8.6 (mean) | 8-18 |
| Dose, mg/kg/d | 2-3 | 2.5 | 2.4 (normal TPMT) 1.5 (low TPMT) | 1.8 |
| Duration of treatment | Up to 2 y in responders | 6-12 mo | NA | Mean (SD), 10 (5.7) mo |
| Time to evaluate efficacy | 12 wk | 2, 4, 8, 12 wk and every 2 mo thereafter | NA | 12-16 wk |
| Outcome | Excellent 28/48 | Reduced SCORAD in 11/12, mean (SD), 27.7 (8.7) | NA | Complete or near-complete clearance in 7/9 |
| Mean follow-up | NA | NA | NA | 33.1 mo |
| Adverse effects | Hypersensitivity reaction in 1/48. Resolved with suspension of therapy Mild elevation of transaminases in 5/48 (treatment suspended by PCP in 1 case) Nausea and vomiting in 1/48 Treatment suspended in 2/48 because of AEs | Mild gastrointestinal discomfort in 2/12 Mild elevation of transaminases in 1/12 (improved when the dose was reduced) No patients interrupted treatment because of AEs | Laboratory abnormalities in 33/82 (24 in the complete blood count and 11 with increased transaminases) Treatment was interrupted owing to AEs in 6/82 cases (2 with abnormal complete blood count and 4 with increased transaminases) | AEs in 3/11: epigastric pain in 2/11 and increased transaminases in 1/11 Treatment interrupted because of AEs (epigastric pain) in 2/11 |
| Remarks | Monitoring of TPMT activity throughout treatment | Oral PDN was administered in all cases during the first 4-6 weeks. Treatment was suspended briefly in cases of laboratory abnormalities and restarted after further laboratory testing |
Abbreviations: AE, adverse effect; NA, not available; PCP, primary care physician; PDN, prednisolone; TPMT, thiopurine methyltransferase.
Our study is subject to a series of limitations, mainly its small sample size. In addition, it was a retrospective study, and adverse events may have been underestimated if patients were not actively asked about them. For the same reason, it is difficult to evaluate the Investigator Global Assessment scale. Finally, the limited follow-up period prevents us from drawing definitive conclusions about the duration of remission and long-term safety of treatment with azathioprine.
In conclusion, our data confirm the efficacy and tolerability of azathioprine in the treatment of moderate to severe atopic dermatitis in children; therefore, we should include it as part of our systemic treatment options in these patients. While azathioprine does not seem to have serious adverse effects on children in the short term, strict monitoring is mandatory in this age group.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Noguera-Morel L, Knöpfel N, Torrelo A, Hernández-Martín A. Estudio retrospectivo del tratamiento sistémico de la dermatitis atópica grave con azatioprina. Eficacia y tolerancia en 11 pacientes pediátricos. Actas Dermosifiliogr. 2019;110:227–231.