Photodermatoses are skin conditions that are induced or exacerbated by electromagnetic radiation (including visible light, UV light, and infrared radiation) from the sun or artificial light sources. In Part 1 of this series we review current understanding of the pathophysiology of these processes and their classification. We also discuss technical aspects and the basic physics of photobiology and describe the equipment required for photobiologic testing and calibration (light sources and measurement instruments).
Las fotodermatosis son enfermedades de la piel inducidas o exacerbadas por la radiación electromagnética (que incluye la radiación ultravioleta, la luz visible y la radiación infrarroja) emitida por el sol o por fuentes artificiales. En la primera parte de esta revisión expondremos los conocimientos actuales sobre la fisiopatología de estos procesos y su clasificación. Además, realizaremos una serie de consideraciones técnicas acerca de las bases físicas de los estudios fotobiológicos y, finalmente, se detallarán los equipos necesarios para su realización (fuentes de luz, instrumentos de medición y sistemas de calibración).
Photodermatoses are skin disorders induced or exacerbated by electromagnetic radiation—including UV radiation, visible light, and infrared (IR) radiation—emitted by either the sun or artificial sources1 (Table 1). Idiopathic or inflammatory photodermatoses are defined as those photodermatoses whose etiology and pathogenesis are unclear but which are presumed to be related to an immunologic dysfunction that causes an abnormal reaction to certain endogenous allergens. This definition excludes light-triggered or light-aggravated dermatoses that are secondary to genetic or metabolic abnormalities as well as photoaggravated dermatoses. Exogenous photodermatoses are induced by the action on the skin of topical or systemic chemical agents (industrial, cosmetic, or therapeutic) that are activated by sun exposure.
Classification of Photodermatoses.
| Idiopathic (or immunologically mediated) |
| Polymorphic light eruption |
| Hydroa vacciniforme |
| Solar urticaria |
| Chronic actinic dermatitis |
| Actinic prurigo |
| Photodermatoses induced by chemical agents |
| Exogenous |
| Phototoxicity |
| Photoallergy |
| Endogenous |
| Cutaneous porphyrias |
| DNA-repair deficiency disorders |
| Xeroderma pigmentosum |
| Cokayne syndrome |
| Bloom syndrome |
| Rothmund-Thomson syndrome |
| Kindler syndrome |
| Trichothiodystrophy |
| Photoaggravated dermatoses |
| Lupus erythematosus |
| Dermatomyositis |
| Rosacea |
| Psoriasis |
| Seborrheic dermatitis |
| Atopic dermatitis |
| Bullous pemphigoid |
| Pemphigus foliaceus |
| Darier disease |
| Other |
There are considerable geographical differences in the incidence of most of these diseases, and it is important to understand that the core of the current body of knowledge consists almost exclusively of studies carried out in white and Japanese populations. The likely differences between populations with different phototypes that inhabit different geographical areas raise the prospect of a future in which photobiologic knowledge is more globalized and a universally accepted classification system is developed.2 In a recent retrospective analysis conducted in the United States, polymorphic light eruption (PLE) was seen in a statistically significantly higher proportion of African Americans compared with whites (67% vs 41%, respectively).3 This prevalence is much higher than that seen in an epidemiologic study carried out in 6 European countries located from the Mediterranean to Scandinavia (18%)4; the different prevalences found in that study can be interpreted as arising from differences in UV doses as a function of geographical location, as opposed to skin phototype or race. More and better epidemiologic studies will lead to a better understanding of the influence of population-based pigmentary traits on the incidence and severity of photodermatoses and will probably lead to some conditions being reclassified either through the grouping of multiple entities under the same terminology or through the characterization of new diseases.2 Possible geographical changes in the incidence and prevalence of photodermatoses should be recorded and monitored at the global level.
Diagnosis of the various idiopathic photodermatoses is based essentially on clinical manifestations. Each disease represents a particular pathologic response to exposure to light. There are no specific diagnostic tests. Although histologic studies and laboratory tests help to rule out other photoinduced or photoaggravated dermatoses, they generally have little diagnostic or prognostic value.
Photobiologic tests (including phototests, photoprovocation tests, and photopatch tests) can help to characterize each condition by establishing the following: a) the action spectrum responsible for the condition, which is useful for establishing a prognosis and for selecting a course of desensitization treatment with phototherapy; b) the light sensitivity threshold (photosensitivity), established by determining the minimal erythema dose (MED), the minimal phototoxicity dose (MPD) prior to psoralen–UV-A (PUVA) phototherapy, and the minimal urticaria dose (MUD); and c) possible exogenous agents that could theoretically be responsible for the onset and progression of the disease, established by assessing and interpreting the results of photopatch tests.
Photobiologic testing requires specific equipment, namely light sources with different light spectra and known, properly calibrated dosimetries.5 No consensus has emerged and there are no universally accepted diagnostic protocols. Measurable parameters (such as pigmentation, erythema, and the various degrees of whealing) are determined and, often, results are interpreted on the basis of subjective criteria and the experience of each dermatologist. In order to interpret each test, the parameters for assessing the results and the means for doing so (visual or artificial) must be defined beforehand.
Given the important role played by certain idiosyncratic characteristics—such as each patient's pigmentation type and status—it is very difficult to control all variables during photobiologic tests. Furthermore, the clinical utility of these tests often depends on the characteristics of the climate and season to which the patient has been exposed as well as his or her sun-exposure habits (occupational or recreational) and use of photoprotection.
Pathophysiology of Idiopathic PhotodermatosesPolymorphic Light EruptionPLE is a disorder characterized by its clinical heterogeneity (papules, vesicles, eczematous lesions, erosions, and scabs) and the delayed onset of symptoms after exposure to sunlight.6 Because of its delayed onset, PLE was for many years considered to be a delayed-type hypersensitivity response to photoinduced autoantigens.
Some studies have reported that the induction of sensitization and the effector response to dinitrochlorobenzene are less suppressed by UV radiation in patients with PLE than in controls.7,8 However, more recent studies have not confirmed the existence of significant differences between patients with PLE and healthy individuals in the response phase following exposure to sunlight.6
Although the autoallergen responsible for PLE remains unknown, the pathogenic mechanisms of the disease—including abnormal responses to UV light in Langerhans cells, neutrophils, and macrophages—are becoming better understood. In recent years, various in vivo studies in humans have demonstrated that UV radiation induces a depletion of Langerhans cells in the dermis and epidermis, largely due to the increased migration of these cells to the lymph nodes.9–12 In patients with PLE, however, Langerhans cells persist abnormally in the epidermis after exposure to UV radiation. There is also a significant reduction in the number of CD11b+ cells (macrophages and neutrophils), significant producers of the immunosuppressive cytokine interleukin (IL) 10,9 and in the number of type 2 helper T cells.10,11 Additionally, neutrophil infiltration in the skin after exposure to UV radiation is much lower in patients with PLE than in healthy individuals.11 The induction of phototolerance with UV-B irradiation in these patients significantly reduces these abnormal responses in terms of the number, migration, and functionality of these inflammatory cells.12
The above findings support the current theory that PLE is the result of a failure of normal UV radiation–induced immunosuppression. An imbalance between the immunosuppressive and stimulative effects of UV radiation (in favor of the latter) could give rise to delayed-type hypersensitivity reactions to photoinduced endogenous neoallergens. The lower immunosuppressive effect of UV radiation in patients with PLE could explain why the risk of skin cancer is, after adjusting for skin phototype and amount of sun exposure, apparently lower in these patients.13
The chromophore and endogenous antigen responsible for PLE have yet to be characterized, although it is possible that multiple chromophores and antigens are involved. Heat shock proteins have been proposed as possible antigens responsible for PLE.14
Hydroa VacciniformeThe pathogenesis of hydroa vacciniforme is unclear and the chromophores responsible for the disease have not been identified.6 Some cases have been associated with latent Epstein-Barr virus (EBV) infection, and the lesions can progress to lymphoma. Erythrocyte porphyrin levels must be measured in order to rule out erythropoietic protoporphyria (EPP).
Given the histologic similarities with PLE, some authors consider hydroa vacciniforme to be a cicatricial, earlier-onset variant of PLE that improves or resolves during adolescence—in other words, an unusual type of delayed hypersensitivity reaction to a photoinduced autoallergen.
Solar UrticariaSolar urticaria is a very rare type of physical urticaria that is triggered by sunlight, has a chronic course, and can often be difficult to control.15 Wheals usually appear immediately after sun exposure on skin that is not generally exposed to sunlight (neck, upper chest, and dorsum of the feet). The face and dorsum of the hands tend to develop tolerance to the sun (skin hardening).
Although most cases of solar urticaria are idiopathic, the disease can occur in association with immunologic conditions such as atopic dermatitis, PLE (in up to 23% of cases), or chronic actinic dermatitis (CAD) (in 3% of cases).16 Cases of solar urticaria induced by topical medications (such as tar-based preparations) or systemic agents (benoxaprofen, chlorpromazine, progesterone) have been described.17 Solar urticaria is occasionally seen in association with other physical or chronic types of urticaria.17
According to the most widely accepted hypothesis, the absorption of light by 1 or more chromophores in the skin or serum of patients with solar urticaria produces a photoproduct capable of binding to immunoglobulin (Ig) E and to the mast cell membrane, and the ensuing degranulation would lead to an inflammatory wheal-type response. The possibility of transferring this response by injecting healthy individuals with previously irradiated serum from patients with solar urticaria confirms the type I allergic mechanism of the disease.18 The chromophores are as yet unknown, and they can be different in each patient.17 The presence of a circulating photoproduct can be determined by subcutaneously injecting autologous serum previously irradiated at the action spectrum at a dose equal to or greater than the photoprovocative dose.19 Detection of circulating photoproducts could be valuable in the selection of certain treatments (such as plasmapheresis, photopheresis, or polyclonal intravenous immunoglobulins).
From a pathophysiological point of view, there are 2 types of solar urticaria.20 Type 1 is an IgE-mediated hypersensitivity reaction to a photoallergen specific to patients with solar urticaria. Patients with type 1 solar urticaria may or may not be positive for passive-transfer antibodies. Type 2 is an IgE-mediated hypersensitivity reaction to a photoallergen that is not specific to patients with solar urticaria. Patients with type 2 solar urticaria are always positive for passive-transfer antibodies. However, this pathophysiological classification has little practical value because the differential diagnosis test (passive-transfer test) has been abandoned because of risk of infection.
Chronic Actinic DermatitisCAD is an uncommon photodermatosis that includes various entities previously described as actinic reticuloid syndrome, photosensitive eczema, photosensitivity dermatitis, or persistent light reaction.21 This acquired form of eczema is induced by UV radiation (very rarely by visible light) and occurs mainly in elderly patients with a history of extensive sun exposure. CAD typically persists and progresses throughout the patient's life. Many patients with CAD have had a history of chronic eczema, atopic dermatitis, seborrheic dermatitis, contact dermatitis, or airborne contact dermatitis since youth.
The 3 main diagnostic criteria are as follows: 1) a persistent eczematous eruption that first appears in sun-exposed areas and can later spread to covered areas; 2) histologic findings of eczema, with or without lymphomatoid changes; and 3) a decrease in the UV sensitivity threshold (lower MED or anomalous reactions to UV-A radiation).21
The most characteristic histologic findings are eczematous spongiotic dermatitis and a superficial perivascular lymphocytic infiltrate with a tendency towards epidermotropism, initially comprising CD4+ and CD8+ T lymphocytes. In more advanced stages, however, there is a clear predominance of CD8+ T lymphocytes. In patients with CAD, unlike healthy individuals, irradiation of the skin with UV light leads to the activation of keratinocytes—which express major histocompatibility complex (class II human leukocyte antigen [HLA-II]—as well as Langerhans cells and T lymphocytes. Furthermore, the T lymphocytes show a tendency towards epidermotropism as a consequence of the specific pattern of adhesion molecules (prolonged expression of E-selectin, intercellular adhesion molecule 1, and vascular cell adhesion molecule 1).22 These findings, not usually found in healthy skin following irradiation with UV light, suggest that CAD is a type of delayed hypersensitivity reaction similar to allergic contact dermatitis.
Although some patients show sensitization to several allergens used in patch testing (topical antibacterial agents, fragrances, whitening agents, etc.), several authors now believe that CAD is the result of an initial reaction to an endogenous photoallergen rather than to an external agent.21,23 The direct action of UV radiation would cause a structural change in the chromophore (presumably DNA or DNA fragments) that would initiate the eczema effector phase or produce oxidative changes in certain normal proteins in the skin, which would then act as endogenous allergens.21 This has been demonstrated in vitro by means of photooxidation of the histidine component of albumin.23
Chronic sun damage to the skin in elderly patients can lead to a UV-induced reduction in immunosuppression (as in PLE) as well as a decrease in the skin's capacity to eliminate antigens; this could explain why these patients are predisposed to developing chronic eczematous conditions. These events could, in turn, predispose these patients to developing autoreactive dermatitis.
Actinic PrurigoActinic prurigo (AP) is a photodermatosis that is especially prevalent in Latin American mestizo populations and in some American Indian populations of North America (Inuit, Navajo, and others) and of Mesoamerica (Mexico, Central America, and northern South America).24
AP was originally considered to be a variant of PLE. However, certain clinical characteristics of AP, and the fact that onset occurs in the first years of life, support the notion that AP and PLE are distinct entities.24,25 AP primarily affects the face (especially the lips) and very often coexists with characteristic chronic conjunctival manifestations. The lesions are characteristically lichenoid and generally no vesicles are present. The most relevant histologic findings are a dense infiltrate in the upper dermis and the presence of lymphoid follicles. Furthermore, the lesions are persistent and do not always disappear in the winter.25 The excellent response to thalidomide—which can also modify the immune response in these patients—is another feature that differentiates AP from PLE.
Autoimmunity studies should be carried out to rule out lupus erythematosus in these patients.
In vitro proliferation studies have demonstrated autoimmune reactivity.26 The lymphoid infiltrate is predominantly characterized by CD4+ lymphocytes and the secretion of IL-2. The keratinocytes show signs of immune activation and production of tumor necrosis factor α (TNF-α). There is a clear relationship between the prevalence of AP and certain HLA antigens in each geographical region.27 It has even been suggested that some HLA peptides could play a role in the pathogenesis of AP.
All of the above findings suggest that AP is an inflammatory process triggered by UV radiation that causes the keratinocytes to release TNF-α, generating an inflammatory response in genetically predisposed individuals.
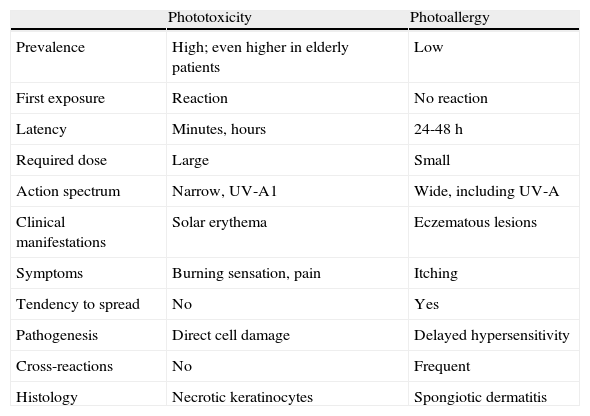
Exogenous PhotodermatosesExogenous photodermatoses are more common in elderly patients and in patients taking multiple medications.28 There are 2 distinct clinical patterns that correspond to different pathogenic mechanisms: phototoxicity and photoallergy (Table 2).
Differential Diagnosis Between Phototoxicity and Photoallergy.
| Phototoxicity | Photoallergy | |
| Prevalence | High; even higher in elderly patients | Low |
| First exposure | Reaction | No reaction |
| Latency | Minutes, hours | 24-48 h |
| Required dose | Large | Small |
| Action spectrum | Narrow, UV-A1 | Wide, including UV-A |
| Clinical manifestations | Solar erythema | Eczematous lesions |
| Symptoms | Burning sensation, pain | Itching |
| Tendency to spread | No | Yes |
| Pathogenesis | Direct cell damage | Delayed hypersensitivity |
| Cross-reactions | No | Frequent |
| Histology | Necrotic keratinocytes | Spongiotic dermatitis |
Phototoxicity, the most common form, does not require a sensitization period. Therefore, it can appear in any individual following first exposure, depending on the dose of the exogenous agent and of UV radiation. Clinically, the lesions resemble a sunburn and are very intense; however, they are generally triggered by an amount of sun exposure that would be insufficient to cause such skin damage. They are the result of cell damage to the skin induced by photoactivated toxic agents, some of which act by means of a photodynamic mechanism to generate reactive oxygen species (including tar-based preparations, nonsteroidal anti-inflammatory drugs, furosemide, and chlorpromazine). Other possible mechanisms of cell damage include the generation of stable photoproducts (as in the case of tetracyclines and quinolones), the formation of photoadducts (as occurs with psoralens in PUVA treatment), and the production or direct release of inflammatory mediators (as has been shown with demeclocycline and chlorpromazine).
Much less common is photoallergy, which is caused nearly exclusively by topical exposure to medications or cosmetics. Clinically, photoallergy is characterized by an acute, highly pruritic eczematous reaction in areas exposed, generally, to a small amount of light following the application of the causative agent. The mechanism is the same as that of contact eczema, except that the allergen is a photoproduct. Photoallergy requires a sensitization period of 7 to 10 days after first exposure.
Technical Aspects of Photobiologic TestingPhotobiologic tests are carried out on a patient's skin in order to determine both the degree of sensitivity to light and the electromagnetic spectral bands responsible for the appearance or inhibition of various anomalous cutaneous responses.
The Electromagnetic Spectrum and the Measurement of LightIn order to understand the units in which electromagnetic radiation is measured, various premises must be considered. First, we must differentiate between the purely physical—that which is independent of human perception—and that which depends on human perception because it is received by the visual system, where the aspects of brightness and color arise.
In the measurement of radiation, in the purely physical sense, we must differentiate between 2 levels: 1) the quantity of energy units associated with the photons, and 2) the number of quantum units, or photons, that interact with a detector. Radiation quantities can be physically measured either radiometrically (energy levels) or photometrically (number of photons per unit of time).
Proper use of artificial light sources requires knowledge of the distribution on the electromagnetic spectrum of the radiation emitted by each light source. UV radiation (100-400nm) falls within the nonionizing portion of the electromagnetic spectrum.
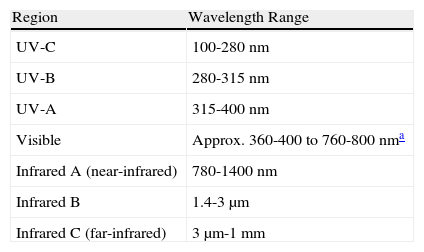
In the spectral band classification scheme used in medicine and biology, the boundary between UV-B and UV-A radiation has traditionally been established at 320nm, as this is the boundary between wavelengths that cause erythema (< 320nm) and those which do not. More recently, separate ranges have been proposed for UV-A1 (> 340-400nm) and UV-A2 (320-340nm). However, in 1987 the International Commission on Illumination (CIE) established standard ranges for each spectral band of UV, visible, and IR radiation (Table 3).
Spectral Regions From Ultraviolet to Infrared, According to the International Commission on Illumination.
| Region | Wavelength Range |
| UV-C | 100-280 nm |
| UV-B | 280-315 nm |
| UV-A | 315-400 nm |
| Visible | Approx. 360-400 to 760-800 nma |
| Infrared A (near-infrared) | 780-1400 nm |
| Infrared B | 1.4-3 μm |
| Infrared C (far-infrared) | 3μm-1 mm |
Radiance, also called radiant flux or radiant power, is the amount of electromagnetic energy emitted by a radiator per unit of time. It is measured in watts (W = J · s-2).
In the case of energy incident on a surface (skin, for example), the term irradiance or radiant flux density refers to incident power per unit of surface area. It is expressed as W · m-2 or, more commonly in dermatology, as mW · cm-2 or mJ · s-1 · cm-2.
The term radiation dose refers to the cumulative irradiance over a period of time. This concept allows us to manage the patient's exposure to a light source and determine the minimum incident energy threshold that produces lesions. Radiation dose is expressed as J · cm-2.
Dose = irradiance (mJ/s · cm) × exposure time (s) = mJ/cm2.
When electromagnetic radiation is measured in quantum terms, the amount of light is measured as the number of quanta (photons) per unit of surface area (m-2) and unit of time (s-1). The term for this unit of measure is photon flux density or electron flux density.
Action Spectrum vs Absorption Spectrum. Biologically Effective IrradianceBecause the skin is the organ that receives incident electromagnetic energy, in cutaneous photobiology it is necessary to understand purely physical concepts of light measurement as well as radiation terms in a biological context. Different types of radiation have different skin-penetration properties, and only the radiation that is absorbed by tissue is considered to be effective.
The amount of radiation absorbed in the skin at each incident wavelength capable of producing a biological effect (by means of a photochemical reaction) is called the action spectrum.
Molecular species in the skin that are capable of absorbing the action spectrum, giving rise to the photochemical reactions that constitute the biological effect of light, are known as chromophores. One of the basic objectives of cutaneous photobiology is to identify the chromophores responsible for particular biological reactions in the skin. However, most chromophores responsible for idiopathic photodermatoses are currently unknown because of the complexity of the tissues, the overlapping of chromophores, and the changes in the physical and chemical characteristics of the chromophores as a result of interaction with other molecules.
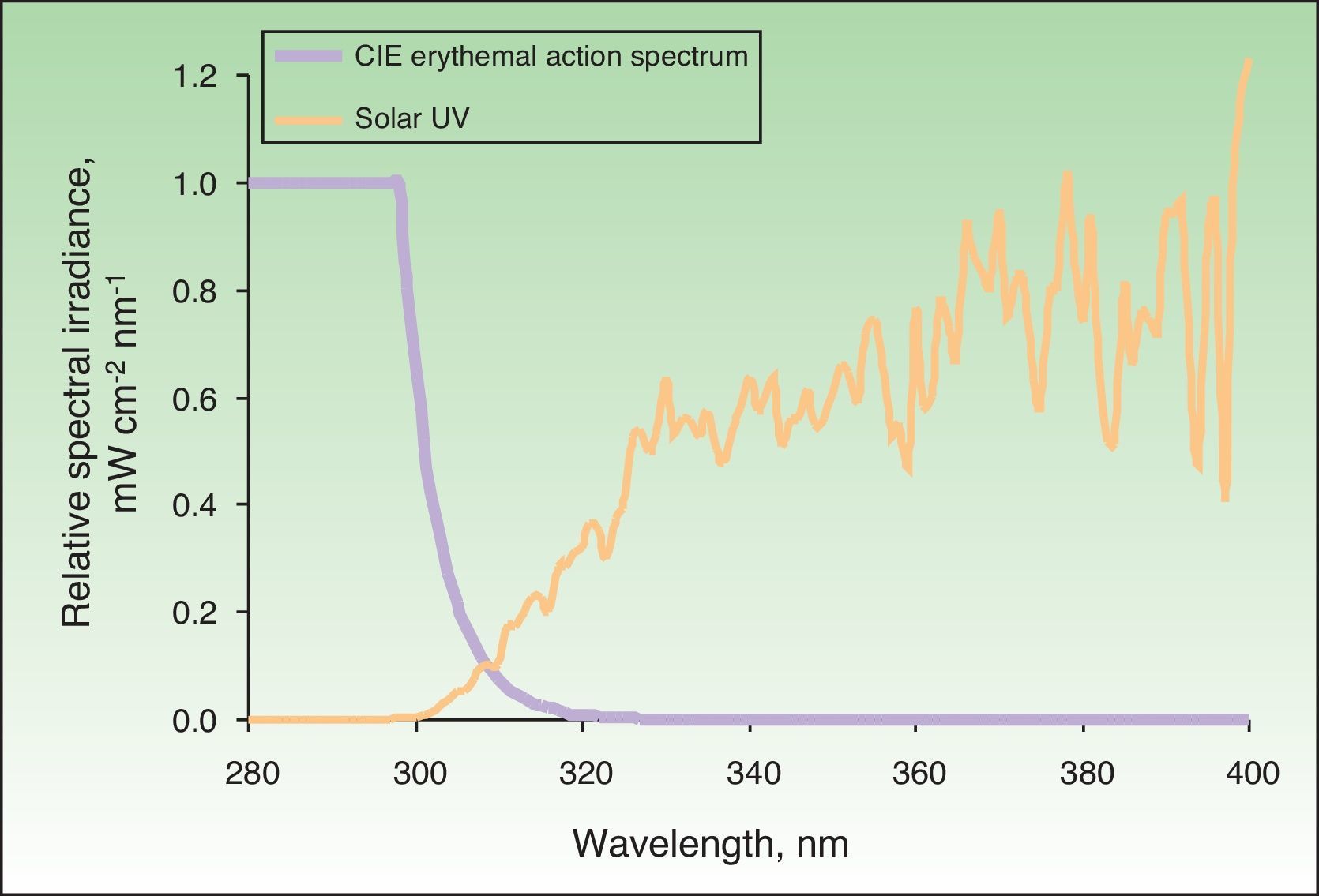
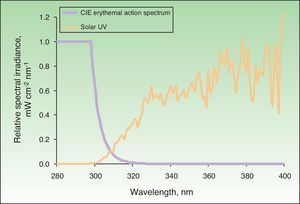
The action spectrum of a particular biological effect is primarily useful for calculating biologically effective irradiance—in other words, irradiance weighted according to the potential biological effect of each wavelength in the spectrum of a particular light source. This makes it possible to determine the effective fraction or quantity for a given effect, which, when multiplied by the exposure time, gives the biologically effective dose. The action spectrum for erythema was determined in 1987.28Fig. 1 shows, in relative units, the solar UV spectrum that reaches the earth's surface and the erythemal action spectrum.
Solar spectrum and erythemal action spectrum. Only a small part of the electromagnetic spectrum is responsible for the appearance of erythema. If we multiply the incident solar energy in each wavelength by the erythemal potential of that wavelength based on the action spectrum (in the figure, this is shown as relative units with a maximum value of 1), we obtain the biologically effective irradiance for erythema (nearly 100% is due to UV-B radiation). CIE indicates International Commission on Illumination.
When the skin is exposed to a source of radiation for a particular period of time, the amount of radiation received, corrected for the erythemal effect, is called the erythema dose of radiation. The minimal erythema dose (MED) is the dose of radiation emitted by a light source, weighted for the erythemal effect of each wavelength capable of causing appreciable, uniform skin redness with well-defined borders. Thus, the MED of any light source can be calculated from the irradiance of the radiation emitted by the source and the effective irradiance that causes erythema (Fig. 1).
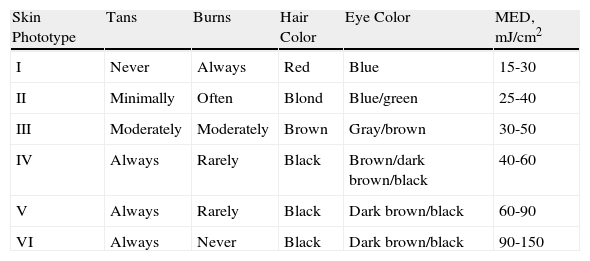
Skin PhototypesBecause the skin possesses particular optical properties as a result of its physical constitution, a change in the physical structure of the skin (thickening of the stratum corneum) or an increase in the number of radiation-absorbing molecules (chromophores such as melanin) can cause MED values to change.29 Therefore, in addition to interindividual differences in photosensitivity derived from race-related skin characteristics or degree of tanning, up to fivefold differences in MED can be found at different body sites in a single individual.30
In order to standardize the types of skin responses to sunlight, the Fitzpatrick31 phototyping scale was proposed in 1988 and, with the aforementioned limitations, standard MED values were established for each phototype (Table 4). However, given the inter- and intraindividual differences in photosensitivity, there is no unambiguous linear correlation between phototype and MED, and neither age nor sex appear to influence MED.32
Phenotypic Characteristics by Skin Phototype and Correspondence to Minimal Erythema Dose.
| Skin Phototype | Tans | Burns | Hair Color | Eye Color | MED, mJ/cm2 |
| I | Never | Always | Red | Blue | 15-30 |
| II | Minimally | Often | Blond | Blue/green | 25-40 |
| III | Moderately | Moderately | Brown | Gray/brown | 30-50 |
| IV | Always | Rarely | Black | Brown/dark brown/black | 40-60 |
| V | Always | Rarely | Black | Dark brown/black | 60-90 |
| VI | Always | Never | Black | Dark brown/black | 90-150 |
Specific equipment is required for photobiologic testing. Firstly, light sources spanning various emission spectra are needed. Secondly, to control the emitted light, radiation measurement equipment is needed in order to calibrate the light sources and, once the spectrum and emission power are known, to define photoprovocative doses for diagnostic purposes. Finally, to ensure the proper use of photodiagnostic units, equipment operators must have specific knowledge of the handling of electromagnetic radiation, the principles behind the operation of the equipment, and the terminology employed in the measurement and handling of electromagnetic radiation.
Light SourcesThe nature of radiation depends on the physical structure of the radiator or light source emitting it. On the basis of these criteria, radiation sources can be classified in 3 groups.
Thermal Radiators or Incandescent LampsThermal radiators are solid elements (such as the tungsten filament of a light bulb) that, when heated to around 2800°C, generate a continuous emission spectrum. One example would be the lamp of a slide projector, which emits a continuous spectrum in the visible range and is used to analyze the spectral bands responsible for solar urticaria.
Discharge LampsPassing an electrical current through a highly pressurized gas excites the gas molecules; as the molecules return to their ground state, energy is released in the form of electromagnetic radiation. The emission spectrum depends on the type of gas (xenon, neon, etc.); each gas has characteristic peaks and bands. This category includes most continuous-spectrum radiation sources in the UV-visible range. The types of discharge lamps used in UV-visible photodiagnosis are solar simulator lamps, monochromators, and high-pressure UV-A lamps. The differences between these light sources lie in their emission spectra.
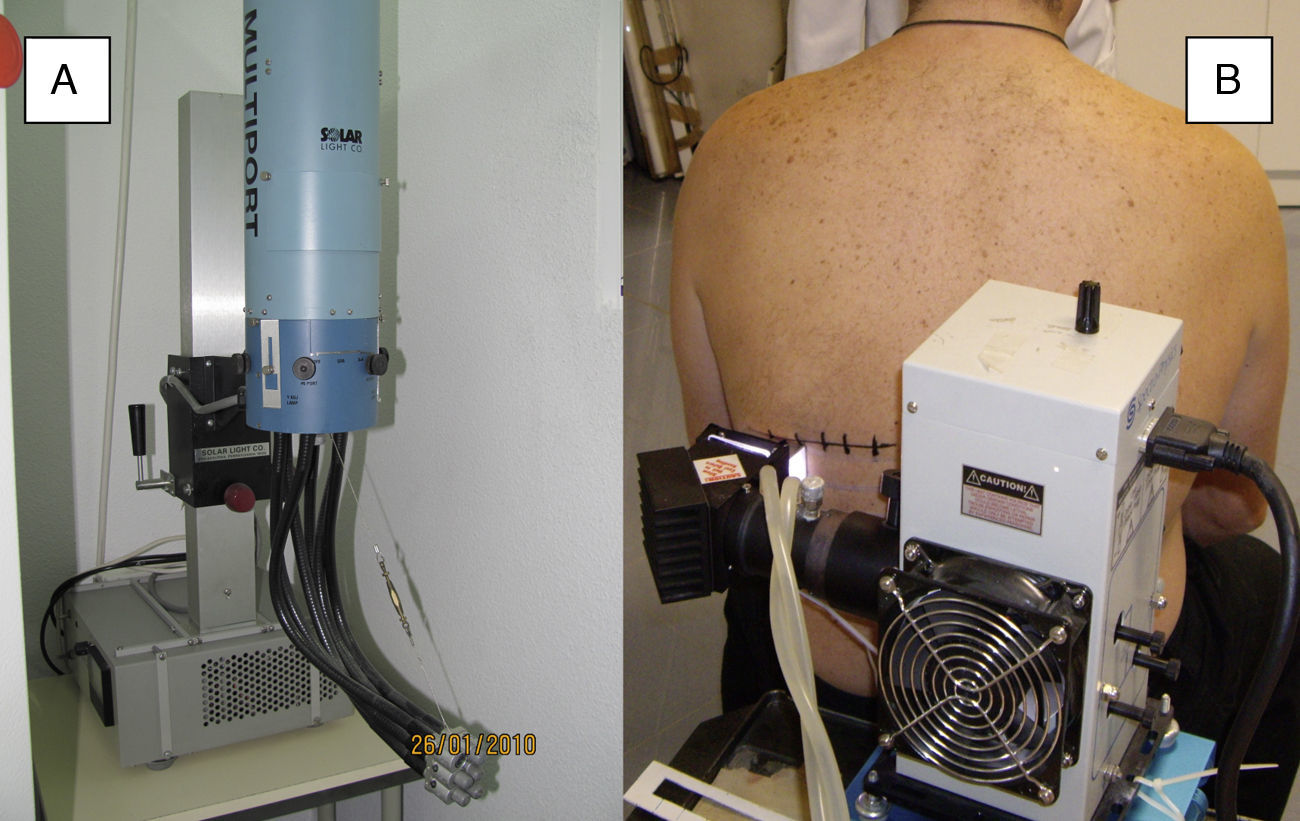
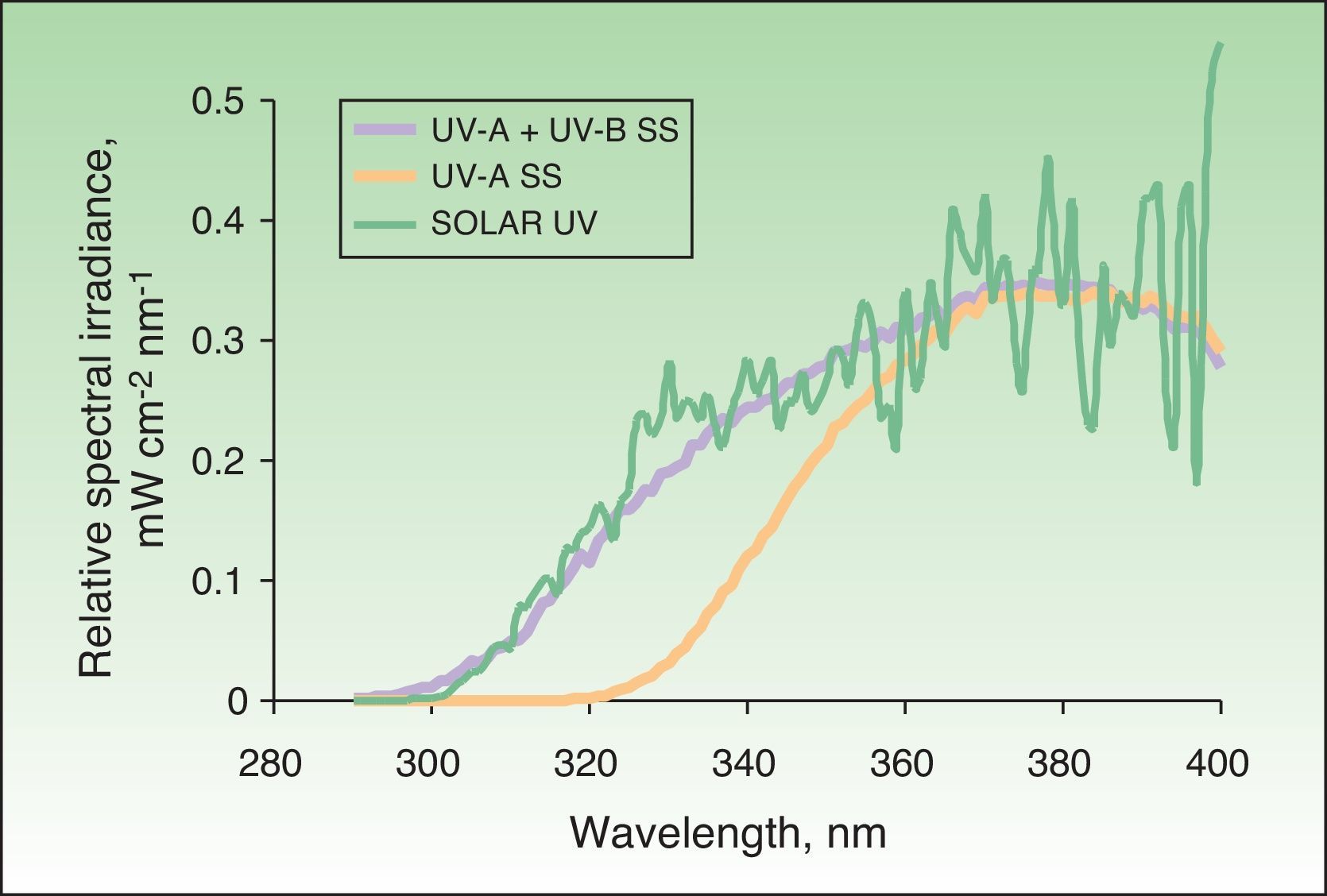
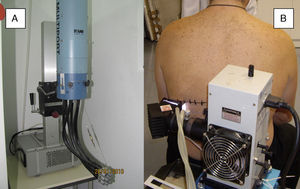
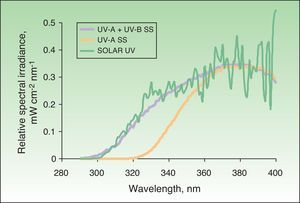
Solar simulatorsSolar simulators consist of a light source (generally a xenon lamp), a collimator, and an optical system that allows the operator to select the emitted light range using a system of internal filters (to eliminate UV-C radiation and visible light) and external filters (to eliminate UV-B radiation at the operator's discretion) (Fig. 2). Solar simulators reproduce solar radiation at the earth's surface (Fig. 3). In cases involving a combination of various chromophores, the selection of cutoff wavelengths in a solar simulator allows a better selection of the spectral bands responsible for the photodermatosis in question. The largest problem with solar simulators is that they undergo changes in spectral emission over time and must therefore be periodically calibrated using a spectroradiometer.
Solar simulators. A, Multiport solar simulator. An increasing dose of solar-simulated radiation is emitted through optical fibers connected to each of the 6 ports to produce erythema simultaneously (each output delivers a different controlled intensity). B, Solar simulator. The lamp and filter system concentrates all of the radiation emitted by the lamp on a single point (1cm diameter) and increasing doses are obtained by increasing the exposure time at each point.
A monochromator is a continuous (heterochromous) light source equipped with a dispersion system (grating or prism) that splits the spectrum of light into multiple very narrow bands, the width of which can be selected by the operator using cutoff filters. A monochromator can isolate specific spectral bands in order to determine which bands are responsible for triggering or inhibiting a particular disease. Monochromators are very useful for determining the action spectra of different photodermatoses.
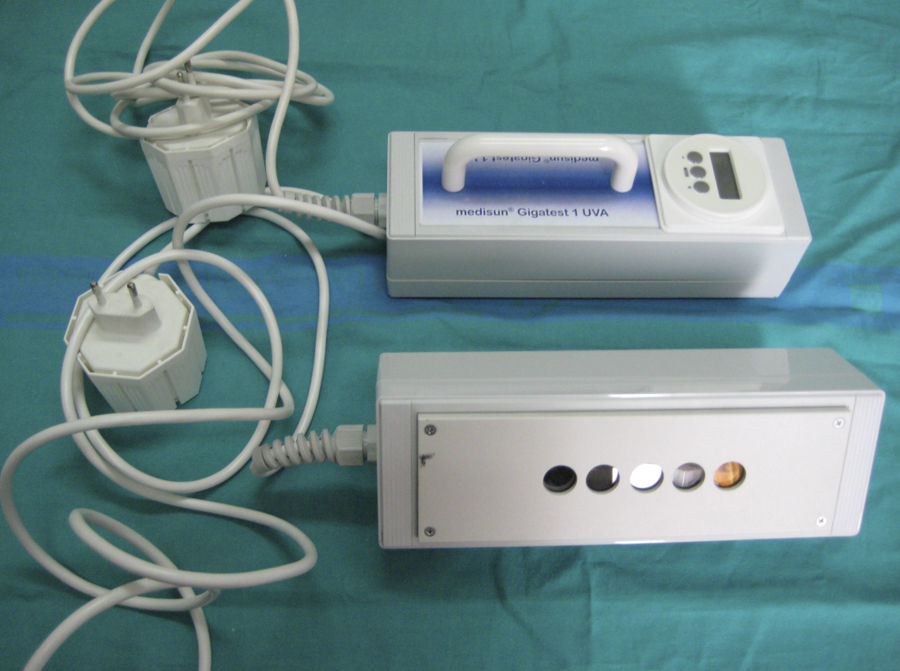
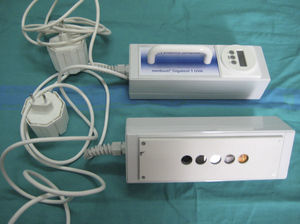
Fluorescent LampsFluorescent lamps contain a low-pressure metallic gas (generally mercury vapor) that, when exposed to an electric current, produces shortwave UV radiation, which then excites the phosphor coating on the inside surface of the bulb. A return to the ground state occurs when the fluorescent coating, in turn, emits light radiation at longer wavelengths (UV-B, UV-A, or visible light). UV fluorescent lamps are commonly used to perform photopatch tests, targeted phototherapy, or full-body phototherapy. Portable fluorescent lamps are useful for measuring UV-B and UV-A MED values and for assessing the action spectrum and MUD of solar urticaria (Fig. 4).
Portable fluorescent hand lamp. A 9-W continuous-spectrum lamp (PLS Special model) with integrated emission spectrum filter, five 5-mm test fields that emit 7%, 34%, 63%, 85% and 100% of the UV-B or UV-A spectrum and therefore make it possible to calculate the exposure dose (in J/cm2) as a function of skin contact time (Gigatest, Medisun, France).
Measuring equipment is necessary for characterizing both the emission spectrum of a light source and the amount of energy emitted per unit of time.
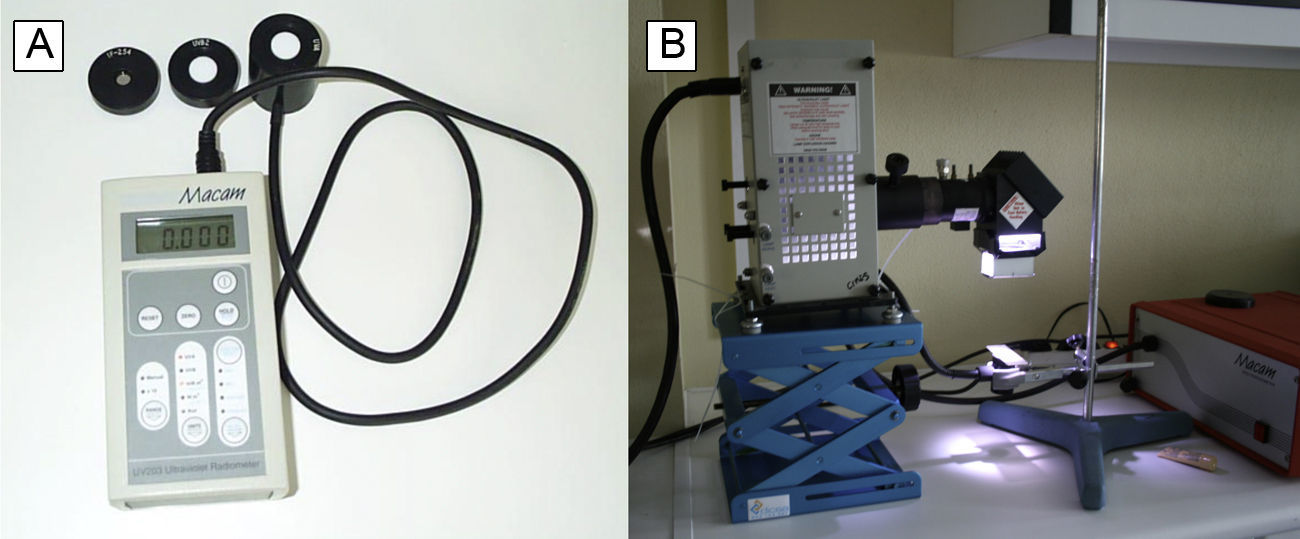
Broadband RadiometersBroadband radiometers contain a sensor with a semitransparent window that allows the entry of photons at the wavelengths being measured (for example, in the UV-B range of the electromagnetic spectrum). The photons are then detected by a photovoltaic detector, which converts them into an electric signal (Fig. 5). On the basis of factors determined by the manufacturer, the electric signal is converted into the photometric units of irradiance (W · m-2) or photon flux (moles of photons · m-2 · s-1). Therefore, a broadband radiometer returns an integrated measurement result for the entire spectral band that the detector is capable of measuring.
SpectroradiometersSpectroradiometers are devices for measuring light radiation that are capable of separating the various wavelengths and determining the emission spectrum of any light source at intervals measured in nanometers. These devices contain monochromators, which are light sources with a dispersion system (prisms) that separates the light into very narrow wavelength intervals.
Equipment Calibration and MaintenanceAll light sources must be spectrally characterized (by means of radiometry or spectroradiometry). It is of fundamental importance to know the irradiance of a given light source at each wavelength, primarily in order to characterize the potential biological effect being measured. The spectral distribution of a light source is normally provided by the manufacturer.
It is essential to have radiometers for measuring the radiation (UV-B and UV-A) emitted by each light source in order to control the emission and doses used for each patient. Ideally, measurements should be taken just before irradiation in order to guarantee that the patient receives the prescribed dose. This is important because certain light sources, including solar simulators and monochromators, contain complex spectral separation systems and can easily become decalibrated. Simpler light sources, such as fluorescent lamps, emit light more uniformly over time. With such lamps, calibration should be performed monthly because the age of the device significantly affects both its emission power and its spectral quality.
The advantages of radiometers are ease of use, measuring speed, and cost. The disadvantages are the need to calibrate the radiometer at least once a year according to spectroradiometric measurements for each of the light sources used in the laboratory (CIE, 1983).
Ethical DisclosuresProtection of persons and animalsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe author declares that no patient data are disclosed in this article.
Right to privacy and informed consentThe authors declare that no patient data are disclosed in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: De Argila D, Aguilera J, Sánchez J, García-Díez A. Estudio de las fotodermatosis idiopáticas y exógenas. Parte I: fisiopatología y aspectos técnicos del estudio fotobiológico. Actas Dermosifiliogr. 2014;105:112–121.