Vitiligo is a skin condition characterized by white, hypopigmented macules. Melanocyte loss is a feature of the disease, and it has been hypothesized that an autoimmune mechanism could be responsible for the depigmentation. Melanoma is a malignancy that develops in melanocytes; if not detected and treated early, it is often deadly. Leukoderma, a condition characterized by depigmentation of the skin, is sometimes associated with malignant melanoma. An immune response against melanocyte antigens leading to destruction of either melanoma cells or melanocytes has been observed in both vitiligo and melanoma. Studies in animal models and humans have shown that humoral and cell-mediated immune responses are involved in modulating cytotoxic activity against tumor cells and normal melanocytes. The study of factors associated with anti-tumor immunopathogenic mechanisms —autoimmunity for example—may provide us with tools for the diagnosis and treatment of diseases such as vitiligo and malignant melanoma.
El vitíligo es una patología cutánea que se manifiesta en forma de manchas hipocrómicas y acrómicas. Se caracteriza por la pérdida de melanocitos y se ha hipotetizado que un mecanismo autoinmune podría estar estrechamente relacionado con este fenómeno de despigmentación. El melanoma es una neoplasia maligna derivada de los melanocitos, que es letal si no se trata oportunamente. La leucodermia es un fenómeno de despigmentación cutánea, que ocasionalmente se puede asociar a melanoma. Tanto en los pacientes con vitíligo como con melanoma se ha observado una respuesta inmune contra antígenos de las células melanocíticas, ya sea para la destrucción de los melanocitos normales como de las células tumorales. A través de diversos estudios en humanos y modelos animales se ha observado que, tanto la inmunidad humoral como la celular tienen un papel inmunorregulador en la citotoxicidad contra el tumor o contra las células melanocíticas. El estudio de los factores asociados a los mecanismos de inmunopatogenicidad antitumoral, así como a la autoinmunidad es, en potencia, una vía alternativa para el diagnóstico y tratamiento de patologías como el vitíligo y el melanoma maligno.
Vitiligo is a disorder characterized by well-delimited white, hypopigmented macules with no detectable melanocytes. The condition is usually acquired, although congenital and familial cases have been reported.1 The main burden of the disease is its effect on the psychological state of the patient. The worldwide prevalence is estimated to be 0.5%2 and the incidence is approximately 1% to 2% in the general population. Clinically, vitiligo presents as round or oval white, hypopigmented macules with regular borders. It can sometimes show a trichrome coloration (band of tan color between the white macule and healthy skin) or quadrichrome coloration (perifollicular or marginal macular hyperpigmentation in cases of repigmenting vitiligo). Another clinical form is inflammatory vitiligo, with a raised red border similar to pityriasis versicolor. The distribution patterns of vitiliginous lesions include focal vitiligo (isolated lesion), segmental vitiligo (unilateral macular lesions which generally cover a dermatome), generalized vitiligo (most common form, disseminated macules of variable size, usually with a symmetric distribution and a certain predilection for extensor surfaces), and vitiligo universalis (severe form that affects more than 80% of the body surface).
Certain proteins located in melanosomes are required for the synthesis of melanoma, the pigment responsible for color in eyes and skin and its appendages.3 These molecules are denoted melanosomal proteins and are classified into 2 groups:
Tyrosinase and tyrosinase associated protein 1 (encoded by the TYRP-1 gene) and protein 2 (encoded by the TYRP-2 gene), which catalyze the biochemical steps in the biosynthesis of melanin
Melanoma-antigen recognized by T cells (MART-1), Pmel17, Rab7, and Rab27, responsible for retaining the melanosomal structures and/or transporting the melanogenic proteins or melanin pigments
All these proteins are important from the pathogenic, diagnostic, and therapeutic point of view in a large number of pigmentary disorders (vitiligo, piebaldism, melanocytic nevus, and melanoma, among others).
To date, different theories have been put forward to explain the possible pathogenesis of vitiligo, with the immunology-based theory being the most widely accepted. On the one hand, it has been shown that vitiligo can be associated with other autoimmune disorders, especially autoimmune thyroiditis. On the other, in these patients, antibodies against melanocyte antigens such as tyrosinase, TRYP-1, and TRYP-2 have been detected. In addition, there are studies that show that cellular immunity also has an impact on the development of vitiligo. Infiltrates have been found to contain CD8+ T cells that are reactive with melanocyte self-antigens such as Melan-A/MART-1, tyrosinase, and gp-100.1
Treatment for vitiligo depends on the presence and severity of associated comorbidities. Therapy is based on topical immunosuppressive agents (corticosteroids and calcineurin inhibitors) and phototherapy to induce repigmentation. In cases of extensive depigmentation, depigmentation of healthy skin is indicated using hydroquinone monobenzyl ether.1
Melanoma is a fatal mucocutaneous or ocular neoplasm that can be sporadic or familial. The neoplasm is associated with a range of genetic factors. Other predisposing factors are exposure to intense sunlight associated with sunburn and a clear phototype (Fitzpatrick skin type I and II, mainly), although patients with darker phototypes can also present with the neoplasm. Melanoma accounts for approximately 5% of all cancers in men and 4% in women.4 The incidence of cutaneous melanoma among Caucasians is reported to be increasing by between 3% and 7% each year.5
The clinical presentations of cutaneous melanoma have been widely reported in dermatology and oncology texts, and so the present review will not cover them in depth. It is however important to mention that lesions are usually macular or nodular, with irregular borders, and generally hyperpigmented with a variety of colors ranging from light coffee to blue and grey. Regions of hypopigmentation (regression) may even be present. Some form of recurrence or metastasis (regional or distant) is reported in between 15% and 36% of patients with early-stage melanoma (I and II) during the clinical course of their disease.4 The accepted prognostic factors in the classification of the American Joint Committee on Cancer (AJCC) from 2009 include tumor thickness, level of invasion (only for T1 melanomas), mitotic rate per mm2, ulceration, presence of satellite, lymph node, and pulmonary metastases, high levels of lactate dehydrogenase,6 and antitumor lymphoid response. The latter factor specifically refers to tumor-infiltrating lymphocytes, whose density in the infiltrate is directly proportional to improved prognosis.4 The function of this infiltrate would therefore seem to be to generate an innate antimelanoma immune response, although unfortunately this is insufficient to fully eradicate the neoplasm in many cases.3 Other prognostic factors that have also been taken into consideration are age, sex, anatomic site of the tumor, and regional lymph node involvement.4
Melanoma is a highly immunogenic neoplasm. In other words, it stimulates the immune system to generate a humoral (antibody-mediated) and essentially cellular (cytotoxic lymphocyte-mediated) response to cytoplasmic antigens as well as to the membrane of melanoma cells.3 Melanoma-associated antigens can generally be classified into 2 groups:
Cancer/testicular antigens. These antigens are highly expressed in normal tissue during development, while expression is limited to testicles and the placenta in adults. They are expressed in some types of cancer. This group includes the melanoma antigen family (MAGE), B antigen family (BAGE), G antigen family (GAGE), and New York esophagus (NY-ESO-1).
Differentiation Antigens. These antigens are expressed by both tumor cells and their normal counterparts, but not in other cell types. In turn, this group of antigens is divided into 2 groups. The first group includes melanosomal membrane proteins such as TYRP-1, TYRP-2, Pmel17 (also known as gp100), and MART-1. Gangliosides (GDs) form the second group. These are widely present in melanoma cells and are thought to play a role in cell-cell and cell-matrix adhesion and in growth factor binding, suggesting that they may be involved in the invasive process of the tumor. This group includes GD3, GD2, GM2, GM3, and O-acetyl GD3.
Melanoma therapy depends on the clinical stage. In early stages, treatment is mainly surgical. Interferon alfa (IFNα) has been used primarily as adjuvant therapy in patients with melanoma who are disease-free after appropriate surgery, but with an intermediate or high risk of recurrence. For advanced stages (disseminated melanoma or inoperable locoregional disease), chemotherapy, radiotherapy, and chemoimmunotherapy have been used with agents such as interleukin 2 (IL-2), which enhances the cytotoxicity of antigen-specific T and natural killer (NK) cells in vitro.7 The response rates, although less than 20%, are nevertheless promising.8 Currently, new treatments against therapeutic targets such as KIT9 or BRAF10 are emerging. Another agent that forms part of this generation, the so-called targeted therapy, is the monoclonal antibody denominated ipilimumab (anti-CTLA4).11 Effort has also been made to develop immunotherapy regimens with dendritic cells, whether in monotherapy or enhanced with molecules such as the glycoprotein gp100,12 and methods such as lymphocyte transfer.13 The above approaches reflect the strong interest in melanoma research.
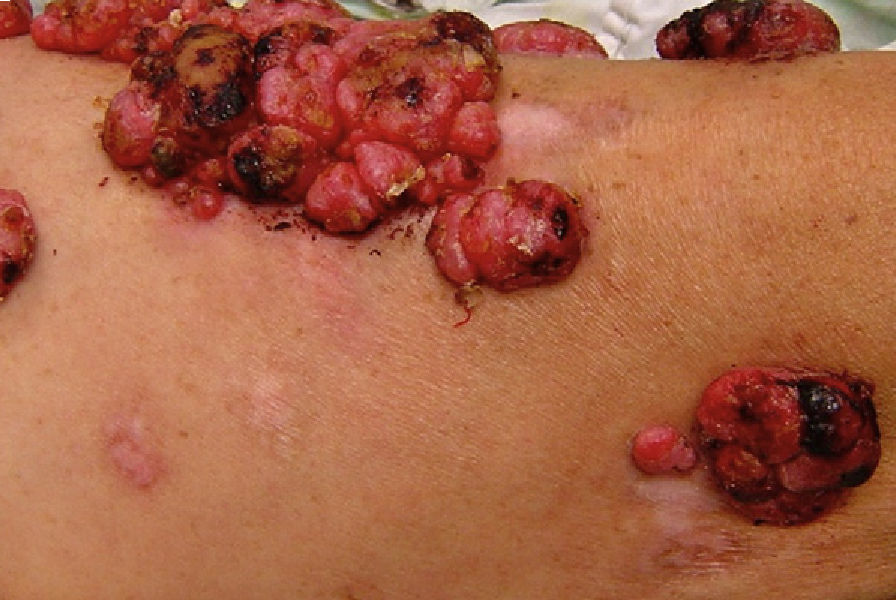
Melanoma-Associated LeukodermaIn certain circumstances, vitiligo (leukoderma) may develop in patients with malignant melanoma, mainly in advanced stages.14 The relationship between melanoma and leukoderma is a controversial and fascinating topic. Around 3% of patients with melanoma may present this melanoma-associated vitiliginous depigmentation.15 Hypopigmentation (leukoderma) in association with benign and malignant melanocytic lesions has been reported frequently with different clinical forms which include regression lesions adjacent to primary melanomas or their metastases (Fig. 1),15–17 nevus with halo (Sutton) around preexisting melanocytic nevi, and vitiliginous lesions at sites distant to the melanoma, whether isolated or associated with ocular findings of the Vogt-Koyanagi-Harada syndrome.2
Melanoma-associated Leukoderma. Multiple pink macules are observed, in addition to hypochromic plaques, some with a cicatricial appearance, adjacent to tumors of an angiomatous appearance (melanoma metastasis) on the anterior face of the left thigh. This patient has been reported previously by Salas-Alanís et al.17
Distant leukoderma may present during the course of melanoma, particularly in cases of advanced stages (metastasis) and the corresponding therapies (mainly immunotherapy-based treatments with IL-2 or IFNα2b).18,19 Longer survival has been observed in patients who develop leukoderma associated with melanoma in advanced stages compared to patients with advanced melanoma who do not develop it,14 suggesting that this is a reflection of the presence of an antitumor activity in the patient. This melanoma-associated phenomenon presents with a frequency of between 1.4% and 20%.2 Unfortunately, as mentioned earlier, the efficacy of this antitumor response is not sufficiently great to increase the chance of complete eradication of melanoma.
Clinically, melanoma-associated leukoderma bears certain similarities with vitiligo. The prevalence of vitiligo among melanoma patients is estimated to be between 3% and 6%.14 In a series of 15 patients with melanoma and hypopigmentation, leukoderma was found to be directly associated with melanoma in 12 cases (80%). Hypopigmentation was observed on average 4.8 years after initial diagnosis of melanoma, and the age of onset was found to be 56.4(10.8) years, in comparison with the age in a group of nonmelanoma associated vitiligo of 27.6 (16.5) years.20 There was no difference between sexes in the frequency of the association. In that series of cases, 75% of the patients with melanoma-associated leukoderma had a bilateral and symmetrical distribution of depigmentation similar to disseminated vitiligo, whereas only 25% of the population studied had a focal or asymmetric unilateral distribution of hypopigmentation; no patients with the association had an acrofacial distribution of depigmentation.20 The progression of melanoma-associated leukoderma is not as fast or progressive as is usually the case with depigmentation in vitiligo. There is no histologic or immunohistochemical difference between the 2 forms of depigmentation. Observations that differentiate between melanoma-associated leukoderma and vitiligo include family history of vitiligo, partial decoloration, and extensive patchy distribution. There is no sufficiently solid evidence to suggest that patients with melanoma are at greater risk of developing vitiligo or vice versa.21 Therefore, given the low frequency of the phenomenon, once diagnosis of cutaneous, mucosal, or ocular melanoma has been made, no special follow-up or greater vigilance is required to detect leukoderma. An appropriate physical examination is sufficient to assess whether hypopigmented lesions are present or not.
The association between leukoderma and melanoma is probably the result of a dual immune response against antigens present in both melanocytes and melanoma cells,2,14 where the primary immunologic effect would be tumor rejection, but with a simultaneous secondary autoimmune effect characterized by hypopigmented macules (leukoderma).8
Role of Humoral Immunity in Melanoma-Associated LeukodermaThe autoimmune (and even antitumor) effects are largely mediated by antibodies against melanocytic differentiation antigens (tyrosinase, TYRP1, TYRP2, and Pmel17). For example, Merimsky et al.22 found significantly higher levels of antityrosinase antibodies in the serum of patients with vitiligo compared to patients with metastatic melanoma, melanoma-associated leukoderma, and healthy controls. They also observed an in vitro inhibition of the proliferation of melanoma cells and a decrease in the incidence and number of metastases in animal models in which animals were treated with immunoglobulin (Ig) G from patients with vitiligo. High titers of anti-TYRP2 IgG antibodies have been found in patients with vitiligo, a similar phenomenon to that observed in patients with melanoma and immunotherapy-induced depigmentation.3 Antibodies against the Pmel17 antigen were also detected in a population of patients with vitiligo using radioimmunoassay.23 In contrast, autoantibodies have not been found in patients with vitiligo.3 Further evidence that humoral immunity and melanoma-associated leukoderma play an important role is the finding that the murine monoclonal antibody TA99 (IgG2a) against the TYRP1/7gp75 protein is associated with hypopigmentation in murine models with melanoma (B16 lineage).8
Although depigmentation can be considered a cosmetic issue, it is interesting to highlight that the immunologic threshold for depigmentation has been shown to be significantly higher than that required for tumor rejection. This is because antibodies can more readily access solid tumors, which have their own vascularization, than melanocytes, which are located in the basal membrane of the epidermis and follicular epithelia.3
In the study conducted by Boasberg et al.24 in a group of 49 patients with metastatic melanoma who were treated initially with concurrent biochemotherapy (dacarbazine, cisplatinum, vinblastine, IL-2, and IFNα2b) and then with maintenance biotherapy based on IL-2 and granulocyte and monocyte colony stimulating factor (GM-CSF), 21 patients (43%) developed vitiliginous lesions. Survival in this subgroup of patients was close to 18.2 months compared to 8.5 months in patients who did not develop leukoderma. High titers of anti-TYRP2 IgG were found in the group of patients with leukoderma (6 out of 21 patients, 29%) compared to patients without depigmentation (4 out of 28 patients, 14%). There were no statistically significant differences in the titers of anti-gp100 antibodies between the subgroups of patients.
Role of Cellular Immunity in Melanoma-Associated LeukodermaAs mentioned earlier, vitiligo lesions contain an inflammatory infiltrate composed of macrophages, dendritic cells, and T lymphocytes, mainly of the CD8+subpopulation,25 although CD4+cells have also been detected. In peripheral blood of patients with progressive vitiligo, as well as in patients with melanoma (after concurrent development of depigmentation), CD8+T cells reactive against MART-1 have been detected. In a similar population, it was found that more than 15% of the T cells in peripheral blood were reactive to the 209-217 epitope of the gp100 antigen. These 2 antigens are considered the most immunogenic of melanocytes. Moreover, T cells that infiltrate melanoma tumors are very reactive against these 2 proteins.3
Ramírez-Montagut et al.8 found that T cells specific for the Melan-A/MART-1 protein were present in 50% of a population of patients with melanoma although there was no correlation with better prognosis. Another study showed the presence of CD8+cells specific for melanocytes in peripheral blood in patients with melanoma, as well as in patients with vitiligo.26 Specifically, these cells were reactive against tyrosinase and Melan-A/MART1 in these groups of patients and most expressed HLA-A*0201.26 The specificity of epitopes that are targeted by CD8+cells suggests that the cells responsible for tumor reduction also cause depigmentation.
In a transgenic melanoma mouse model, almost half the animals (45 out of 88) had permanent leukoderma. In follow-up at 6 months of age, the animals with melanoma-associated leukoderma had significantly fewer facial and dorsal tumors than animals of the same age that had not developed leukoderma. This study also showed that the proportion of peripheral blood lymphocytes specific for melanoma was significantly greater in the subgroup of mice with melanoma-associated leukoderma (1 out of every 680 lymphocytes) than in mice without leukoderma (1 out of every 1000 lymphocytes). These lymphocytes were predominantly CD8+and they also induced secretion of IL-12 and IFN-γ, both of which possess antitumor properties.27 The infiltration of immune cells confers a certain degree of protection in this model, as 140 days after the introduction of Melan-ret syngeneic cells, 25% of the transgenic mice with vitiligo were still protected against melanoma, whereas no leukoderma-free mice were protected. The antitumor reactivity is mediated by CD8+cells specific for TYRP2 and gp100. The same authors have shown the natural induction of cellular immunity mediated by CD8+T cells in humans during the course of melanoma progression and development of leukoderma.27
It is still subject of debate whether the presence of CD8+T cells is the cause or a consequence of leukoderma, as reflected in a study by Byrne et al.28 in which the autoimmune destruction of melanocytes was shown to be necessary for an appropriate and lasting antitumor immune response in a mouse model.
The role of IL-2 is crucial in the development of antitumor cellular immune responses because, in addition to inducing the recruitment of cytotoxic and NK T cells, this cytokine has also been observed to suppress CD4+CD25hi-FoxP3+regulatory T cells (regulatory T cells that are the first antigen-specific T suppressor cells) in patients with metastatic melanoma who respond favorably to the cytokine. Therefore, IL-2 increases cytotoxic function in CD8+cells responsible for both tumor regression and autoimmunity.7
PerspectivesAnimal models such as the Sinclair pig are very valuable for understanding the pathogenesis of melanoma-associated entities. This species is born with congenital melanomas that spontaneously regress a few weeks after birth with the concurrent presentation of generalized hypopigmented macules. An increase was observed in the titers of antibodies against pigmentary cells on presentation of leukoderma.2
The B16 melanoma cell line has been used in mice models to continue the line of investigation into humoral response against antigens of melanocyte differentiation, and it has even been observed that passive immunization with antibodies against TYRP-1/gp75 results in rejection of the syngeneic melanoma and reduction in pulmonary metastases in animal models of metastatic melanoma.8
Lengagne et al.27 established an experimental model in MT/ret transgenic mice with the B6 cell line. The animals in this model develop melanoma lesions at an early age (65% of mice in a 10-month period) and, subsequently, vitiliginous lesions. The authors suggested that the addition of zinc to the water of the mice had induced the development of melanoma.
Cytotoxic T-cell associated antigen 4 (CTLA-4) is responsible for negative regulation of the activated cytotoxic T cells. Blockade of this molecule with a specific anti-CTLA-4 antibody has been demonstrated both in animal models and in humans, with favorable responses. It should be mentioned that the clinical response to treatment is associated with manifestations of autoimmunity, including leukoderma, suggesting that CTLA-4 is important for maintaining peripheral tolerance to antigens.29 These and other molecules will continue to be studied in order to obtain an effective antitumor therapy with limited toxicity, and so improve the quality of life of patients affected by this aggressive malignant neoplasm.
In view of the immunogenic characteristics of melanoma, the development of antitumor vaccines continues to be a challenge. In fact, a vaccine has been developed that contains 6 melanoma-associated peptides, which are derived from the MAGE, MART-1/Melan A, gp100, and tyrosinase proteins. Toxicities associated with immunization include fatigue, headache, myalgia, arthralgia, and nausea. Other types of toxicities were of the autoimmune type, and included elevated rheumatoid factor (10%), treatment-associated leukoderma (10%), and antinuclear antibody elevations.30
The characteristics of the inductive and effective immune response present in melanoma-associated leukoderma lesions, as well as the selective destruction of pigmentary cells in vitiligo, are the points on which further investigation will focus. In theory, therapy involving transfer of immunoglobulins from patients with vitiligo and administration to patients with melanoma would be possible. Self-transfer may also actually induce an antitumor immune response due to the induction of cross reactivity.3 It may even be that the serum of patients with generalized or universal vitiligo could have a more potent antimelanoma effect than that of patients with focal or segmentary vitiligo, and so this is an area of opportunity within the field of immuno-oncology.
Ethical ResponsibilitiesProtection of human and animal subjectsThe authors declare that no tests were carried out in humans or animals for the purpose of this study.
Confidentiality of dataThe authors declare that patient data do not appear in this article.
Right to privacy and informed consentThe authors obtained the informed consent of patients and/or subjects mentioned in this article. The informed consent form is located in the archives of the corresponding author.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: González R, Torres-López E. Bases inmunológicas de la hipopigmentación vitiligoide asociada a melanoma. Actas Dermosifiliogr. 2014;105:122–127.