High-frequency skin ultrasound (HFSU), a relatively new diagnostic technique still under investigation, has been shown to be a useful noninvasive procedure in different areas of dermatology.1,2 The physical principal underlying this technique is the emission of high-frequency ultrasonic pulses (> 10 MHz) by a transducer probe in contact with the skin, which are subsequently recorded by a processing unit as they are reflected back to the probe.
The data generated can be displayed on screen in several modes, labeled A, B, C, etc. In B-mode (one of the most widely used display modes in cutaneous ultrasonography), the processing unit represents the electrical signals as dots of greater or lesser intensity on the screen. The images created represent a longitudinal or transverse section depending on the orientation of the probe relative to the structure under study.1 One important consideration is that the higher the wave frequency the higher the image resolution (defined as the ability of the system to discriminate between the echoes of 2 adjacent structures); however, the converse is true of tissue penetration, which correlates inversely with frequency.1,2 Thus, maximum skin penetration at a frequency of 20 MHz is between 8 and 10 mm, but at 50 MHz penetration decreases to approximately 4 mm and at 100MHz to around 1.5 mm.3 Many modern ultrasound systems are also equipped with combined Doppler probes that can detect and measure the speed and distribution of blood flow in skin structures.1 Although the minimum frequency recommended for the study of skin structures by most authors is 20MHz, the authors of many of the studies in the literature report good correlations between ultrasonic and histologic measurements even with probes emitting frequencies of 15 MHz or lower.2–4
Since the earliest publications on skin ultrasound in the late 1970s,5 the use of this technique has spread to a wide range of applications and is still growing, as shown by the increase in recent years in the number of studies dealing with an ever greater range of diseases. For instance, HSFU has been shown to be useful in the field of inflammatory skin diseases, where it is used to assess the extent of infection, activity in the case of rheumatic diseases, treatment response in psoriasis, among other applications. It is also used in patients with nail disorders (subungual tumors and psoriatic onychopathy), in cosmetic interventions (dermal filler assessment, guided punctures, skin aging studies, etc.), and to assess tumors,1 the focus of the present article.
Many studies have been carried out on the usefulness of HSFU in skin cancer. We do not intend to deal with the studies on ultrasound in melanoma, which have mainly studied the agreement between ultrasound tumor thickness measurements and Breslow depth values obtained by histology.6 The focus of the present article will be the study of non-melanoma skin cancer and specifically basal cell carcinoma (BCC), a field in which HSFU has yielded promising results.
Before discussing the utility of ultrasound in BCC, we will review the current situation and pose some preliminary questions. As is well known, BCC is the most common subtype of non melanoma skin cancer, accounting for 80% to 90% of all cases.7,8 Owing to its high incidence, BCC generates a significant health care burden and high healthcare costs, making it a major public health problem that some authors consider to be of epidemic proportions.9 Management of these tumors is essentially based on the use of invasive interventions (Mohs surgery, conventional surgery) and noninvasive treatments (mainly photodynamic therapy, imiquimod, and cryotherapy).7,8 Choice of treatment will depend on the histologic subtype of the tumor (aggressive or nonaggressive) identified in a sample obtained by incisional biopsy (usually a punch biopsy) and other considerations, such as site, comorbidities, availability of resources, and patient preference.7,8 The invasive techniques are currently the first-line treatment in most cases because they are associated with lower recurrence rates and allow the physician to ensure disease-free margins.7,8,10 However, the growing incidence of this cancer in ever younger populations has led to a higher demand for less invasive treatments that provide better cosmetic results, and noninvasive techniques play a more important role in this setting.10
The first question we must address is whether we are accurately diagnosing the histologic subtypes of BCC in our daily practice and with what degree of certainty, because this information is fundamental to optimum treatment. The consequences of the erroneous classification of an aggressive tumor as a nonaggressive subtype are well known (poor therapeutic management, recurrence, increased health spending, and so on). The literature contains many examples, including very interesting studies that have evaluated diagnostic accuracy by comparing the concordance between diagnoses obtained by incisional and excisional biopsy.10–14 The concordance rates reported range from between 50% to 89% depending on the study,11 and several authors have estimated that an error is made in the histologic classification of subtype in 1 out of 3 cases of BCC11 and 1 out of every 6 cases of BCC with an aggressive histology.10 Other series found that up to 21% of subtypes diagnosed as aggressive after excisional biopsy had been classified as superficial on the basis of the initial incisional biopsy.13 It has been suggested that this high degree of inaccuracy in the preoperative biopsy study is due to the fact that incomplete and misleading results may be obtained because only a very small portion of the tumor tissue is analyzed.11 Logically, the rate of diagnostic success declines with increasing tumor size since the proportion of the tumor analyzed by the biopsy is progressively lower. Success rates are also lower in mixed-component tumors because of the risk that only the superficial component may be analyzed and the aggressive portion may be missed. Thus, although concordance between incisional and excisional biopsies can be considered to be relatively good in tumors of a single histologic type, agreement is considerably lower in mixed-type BCCs (83% vs 37%).11 In fact, it is estimated that around 40% (from 18% to over 50% depending on the series) of the BCCs we encounter in routine practice have mixed histology.10
Thus, incisional biopsy is a technique with limitations when it comes to differentiating aggressive and nonaggressive forms of BCC.12 In clinical practice, underestimation of aggressive variants leads to an erroneous therapeutic approach in most cases, giving rise to tumor recurrence in the long term, with the consequent additional cost to the health system and emotional cost to the patient.10 Many authors now consider poor classification of subtype to be one of the main causes of recurrence in noninvasive therapies such as topical imiquimod and photodynamic therapy. In fact, some studies indicate that the rate of recurrence after treatment of primary BCCs with photodynamic therapy coincides precisely with the rate of erroneous histologic classification based on punch biopsy.11 Moreover, the use of these noninvasive treatments (which are indicated only for nonaggressive BCC subtypes) has increased considerably in the last 10 years, making accurate pretreatment diagnosis even more important.11
It is precisely in this area that HSFU can play a critical role in helping the clinician to differentiate more accurately between tumor subtypes. Although not yet established as a standard diagnostic method in clinical practice, ultrasound has been shown by research to be potentially quite useful in BCC for both tumor measurement (providing information for planning the most appropriate surgical resection) and as a diagnostic technique.15
Most of the published research in this field deals with the study of tumor location and size, delineation of surgical margins, and comparison of ultrasound findings with histologic results obtained following subsequent excisional biopsy of the lesion.16–23
Concordance rates between HSFU and histology for tumor size are generally above 73%, and in some series as high as 98%.15–23 A few studies report poorer results,19,24 but these series also include squamous cell carcinomas, which are more difficult to assess with HSFU (since they frequently present ulceration and hyperkeratosis and these features generate ultrasound artifacts that make interpretation more difficult). Published rates of tumor-free margins in BCC treated surgically following delimitation of margins by HSFU are as high as 95% (although the highest rates have been found when the conditions were favorable, that is, nonaggressive subtypes in easily accessible and low-risk sites.17
It is important to note that compared to histologic measurement HSFU tends to overestimate the extent of the tumor, especially the surface area measurements (length and width); one explanation that has been advanced to account for this discrepancy is the ex-vivo shrinking of the biopsy sample during the process of its preparation for histologic study.17,24,25 The published results for comparison of HSFU measurements with presurgical clinical measurements are inconsistent, although the findings of some recent studies indicate that HSFU yields better results.16,17,24 This would be logical in that ultrasound can detect deeper tumoral spread not susceptible to clinical measurement. HSFU can also visualize underlying cartilage in certain high-risk sites, such as the nose and ear and, by providing information on the extent of such involvement, can prevent incomplete resections.16 HSFU has also proven useful in the detection of subclinical lesions that would otherwise have gone unnoticed.16
The size of the ultrasound probe is one of the major limitations of this technique when it is used to delineate tumor size and margins, particularly in the latter case. The probes are large, making access difficult in certain locations, such as the creases and folds of the nose, ears and eyelids, all of which are high-risk sites for BCC. Similarly, in highly asymmetric lesions or lesions with irregular extensions, it is very difficult to accurately determine the precise outline of the tumor in the patient's skin, a crucial factor in surgical planning particularly in the case of Mohs microsurgery. Altogether, one could say, as many authors have, that although HSFU will not replace histology, it is a useful tool to consider when estimating tumor size, drawing up a surgical plan, or stratifying high-risk patients.16
Less has been published on the usefulness of HSFU in describing the ultrasound patterns that can differentiate between tumor subtypes, an area in which the difficulties have been greater. Wortsman et al.,26 in a retrospective study of 4338 skin lesions analyzed using HSFU (including 75 malignant tumors, mainly BCC), concluded that the technique yields a correct dermatological diagnosis in 97% of cases, with a sensitivity of 99% and a specificity of 100%.
Similarly, Bobadilla et al.16 carried out a prospective study of 25 patients with 29 BCC using a 15 MHz probe. They compared preoperative ultrasound data on both tumor size and presumptive diagnosis with the findings of histology after excisional biopsy. HSFU detected the tumors in all cases and tumor size as measured by ultrasound correlated with histologic measurements in 90%; all the BCC were excised with tumor-free margins. In that study, 27 (92%) of the BCC were nodular and in all cases ultrasound demonstrated the hypoechoic and heterogeneous oval pattern of a solid tumor with irregular borders located in the dermal-epidermal border (consistent with the patterns described by other authors).27 The histologic subtype of the 2 remaining tumors was morpheaform in one case and cystic in the other, but these subtypes were not distinguished by differences in the ultrasound pattern. However, other authors, who describe the same ultrasound findings as Bobadilla et al. for nodular BCC, have reported differentiating ultrasound patterns for other histologic subtypes as well.17 The pattern observed in the case of superficial BCC was similar to that described for nodular tumors but more flattened and not oval. In the morpheaform subtype, increased echogenicity was observed in the area around the main hypoechoic tumor, a finding the authors attributed to the increased fibrosis that characterizes this subtype. In the case of infiltrative BCC, they described hypoechoic bands radiating outwards from the main hypoechoic mass to penetrate the underlying dermis. They concluded that HSFU can be a sensitive screening method for identifying patterns of aggressive growth when the clinical findings are unclear and that it may be useful for planning surgery in high-risk sites, as well as for optimizing treatment and reducing morbidity; these conclusions are also echoed by other authors.28 It should not be forgotten, however, that the ultrasound patterns of aggressive forms of BCC (infiltrative and morpheaform) can mimic the non-aggressive forms (superficial and nodular) and that this could give rise to diagnostic confusion. In such cases, histologic analysis remains the key diagnostic technique.
Studies of BCC using Doppler analysis have also been undertaken and those authors report increased arterial vascularization within and around the tumor, with a predominance of deep vessels.16
Another diagnostic finding described by several authors16,18,28 has been the presence of intralesional hyperechoic spots, which have been studied in greater depth by Uhara et al.29 In a retrospective study, they analyzed 85 cases (29 BCC and 56 melanomas) by correlating histologic and ultrasound findings looking for calcification, cysts, and clusters of apoptotic cells. In conclusion, they proposed that the many large and ill-defined, hyperechoic spots that appear to be characteristic of BCC correspond to numerous large foci of calcification. They also concluded that the presence of such spots could be a useful way of differentiating BCC from melanoma. If this were the case, HSFU could prove to be a useful noninvasive tool for diagnosing BCC.
Following all these lines of research, our department recently conducted a preliminary study on the usefulness of HSFU for differentiating aggressive from nonaggressive patterns in BCC and obtained encouraging results. Using a 20 MHz Dermascan C ultrasound scanner we studied several cases of BCC that recurred clinically after treatment with photodynamic therapy (Figs. 1 and 2). Our findings point to a possible superiority of HSFU over incisional biopsy in detecting infiltrative patterns in mixed-component tumors when the infiltrative portion of the tumor had not been sampled by the incisional punch biopsy.
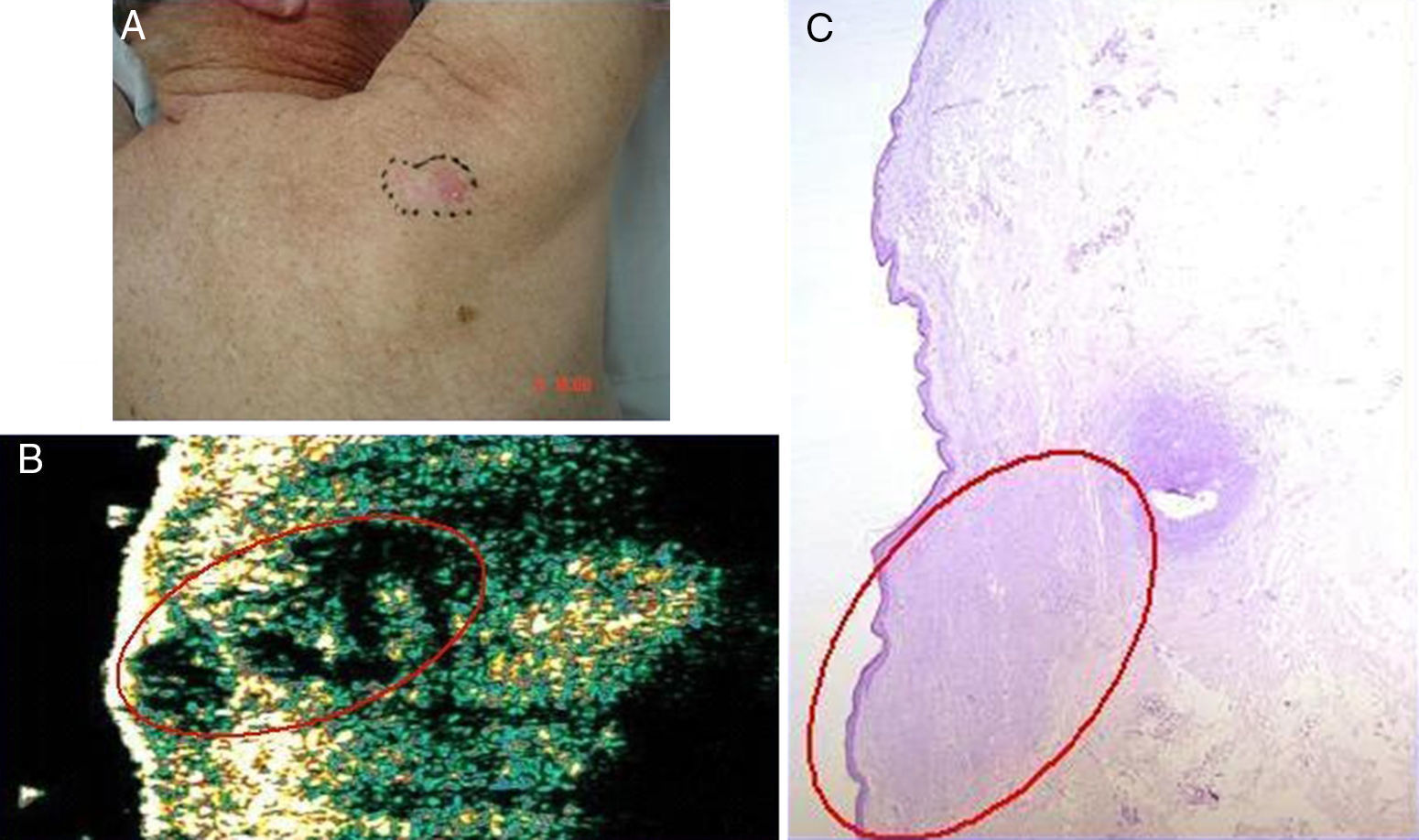
Basal cell carcinoma on the back in a 71-year-old man. A, Clinical image of the lesion. B, Ultrasound image (20 MHz) of the lesion showing a poorly-defined, more or less rounded, hypoechoic subepidermal structure with an elongated and ill-defined hypoechoic prolongation extending into the underlying dermis, suggestive of infiltration. C, Histologic image of the lesion (hemoxylin eosin ×50). The area circled in red indicates the marked infiltrative component of the lesion.
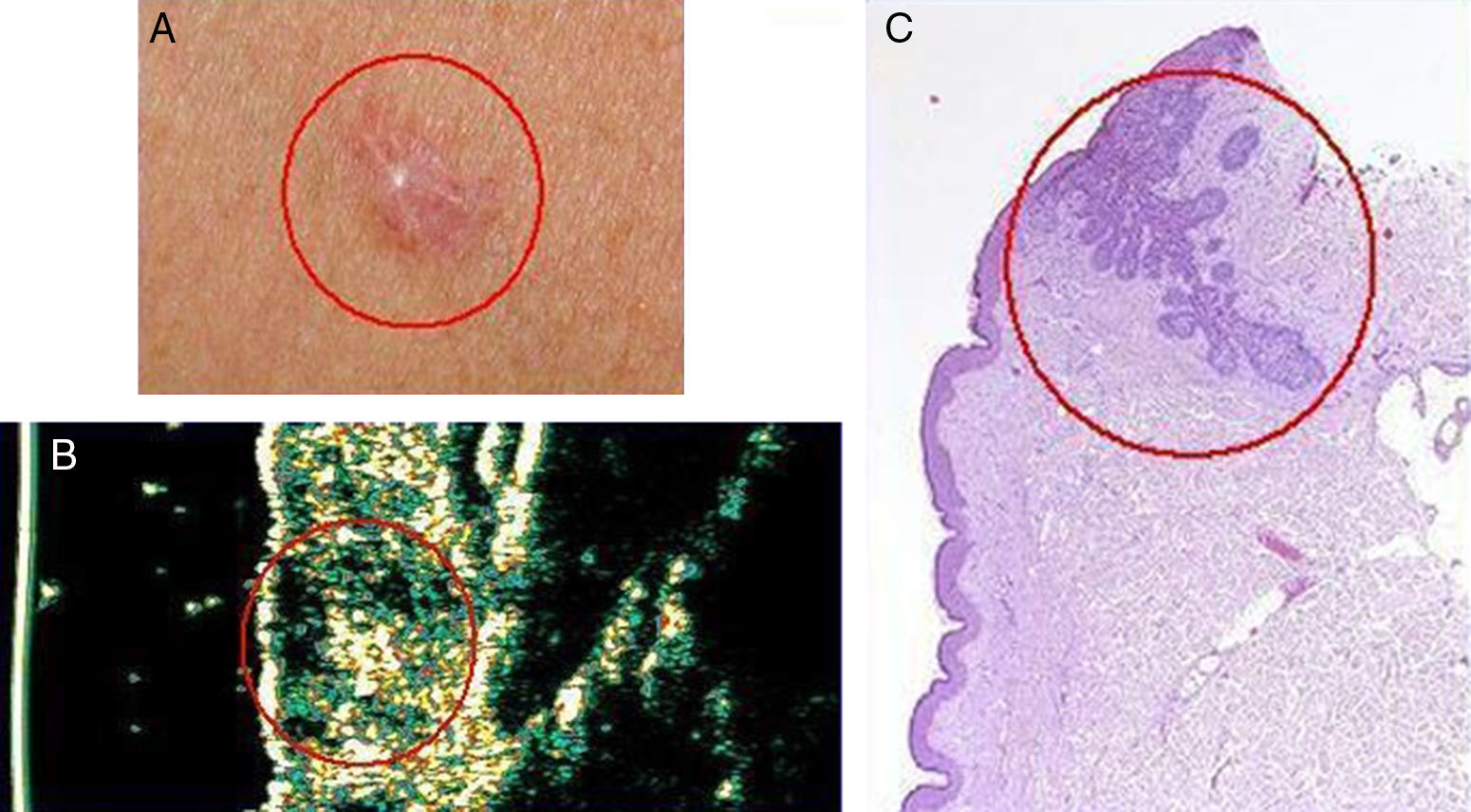
Basal cell carcinoma on the chest of a 50-year old woman. A, Clinical image of the lesion. B, Ultrasound image (20 MHz) of the lesion showing a poorly-defined and flattened subepidermal hypoechoic structure. Note the irregular, ill-defined hypoechoic formation beneath this structure, which is suggestive of infiltration. C, Histologic image of the lesion; the areas circled in red are the infiltrative component of the basal cell carcinoma (hemoxylin eosin ×50).
Finally, we must point out some additional limitations of the technique not mentioned above. Assessment can be difficult in the vicinity of scars or areas of curettage or biopsy. HSFU does not detect tumor aggregates that are smaller than the resolution limit of the ultrasound used. Nor does it differentiate between the tumor and adjacent inflammation, a shortcoming that may lead to overestimation of tumor size. It is difficult to differentiate superficial BCC subtypes in skin with a high degree of elastosis and impossible to determine lesion margins if the dermal-hypodermal border is involved. Furthermore, HSFU is an operator-dependent technique and the operator must have a certain level of experience and training, which involves a learning curve. We should also mention the current high price of high-frequency ultrasound scanners, which may be an obstacle to their acquisition in some cases.
In conclusion we can say that histology may have found a valuable ally although much work remains to be done with HSFU (development of smaller probes and smaller scanners to provide greater functionality, development of digitization techniques, improved resolution, further studies of the technique with a greater number of cases). However, despite current limitations, HSFU is clearly a technique of great potential value for assessing BCC and other tumor and non-tumor lesions. In our opinion—as was the case with dermoscopy only a few years ago—in a relatively short or medium term many hospitals will be equipped with an ultrasound machine for use in daily dermatologic practice.
Conflicts of InterestNone.
Please cite this article as: Hernández C, del Boz J, de Troya M. ¿Es la ecografía cutánea de alta frecuencia una alternativa en el diagnóstico y manejo del carcinoma basocelular? Actas Dermosifiliogr. 2014;105:107–111.