Vaccines against the severe acute respiratory coronavirus 2, which are the first to be used in humans against any coronavirus, were developed and produced in record time. Dermatologic adverse effects appeared during clinical trials and have also been described in the population since approval. Just as descriptions and categorization of skin manifestations of the coronavirus disease 2019 proved important for understanding the disease itself, characterizing the effects of vaccines may also further that goal. This paper reviews the properties of the different types of vaccines currently available and under development and describes how they interact with the immune system and the clinical signs they may cause. We focus on dermatologic adverse effects reported to date and recommendations for managing them.
Las vacunas contra el SARS-CoV-2 son las primeras vacunas que han sido usadas en humanos contra coronavirus y su desarrollo se ha producido en un tiempo récord. En los análisis de seguridad de los ensayos clínicos previos a su aprobación y en la fase postautorización en la población, se han descrito efectos secundarios dermatológicos. La descripción y categorización de las manifestaciones cutáneas de la COVID-19 fueron importantes para el conocimiento de la enfermedad y de la misma forma pueden serlo las generadas por las vacunas. En este artículo hacemos un repaso a las características de los diferentes tipos de vacunas disponibles y en desarrollo, su modo de interacción con el sistema inmune, las consecuentes manifestaciones clínicas que pueden generar, con especial interés en los efectos secundarios dermatológicos hasta el momento descritos, y las actitudes terapéuticas recomendadas ante cada una de estas reacciones.
The lessons learned from other epidemics (Ebola, SARS-CoV, and MERS-CoV) and the international coordination of the knowledge generated have facilitate the global response to the COVID-19 pandemic. The COVID-NMA international research initiative, which arose from the WHO R&D plan, has facilitated the joint evaluation of a large number of clinical trials and of evidence on prevention, treatment, and vaccines for the disease. This has made it possible to gain knowledge, make decisions, and achieve a vaccine, the essential tool for controlling the pandemic, in record time1,2.
Although such vaccines were being developed, to date, no effective vaccine had been used in humans against coronaviruses (CoV). Preclinical development was reached with SARS-CoV but was halted due to the disappearance of the virus as a health threat. But the similarity of the gene sequences of SARS-CoV-2 and SARS-CoV3 reduced the time given over to design. Phase I/II clinical trials soon began and, based on the intermediate analysis of the results phase II trials were begun more quickly and with parallel trials. Before the studies were completed, production of several candidate vaccines had already begun. All this made it possible to have the vaccines available to inoculate the population with unimaginable speed1.
The novel technology used and the speed of the process does not mean that the necessary safety controls were ignored. But it was to be expected, as we are seeing, that with clinical use, adverse effects would appear that were not found or were not sufficiently categorized during the preclinical phase. Cutaneous manifestations and their categorization were important for understanding the disease4, in the same way that those caused by the vaccines may be5. In many of these dermatologic manifestations associated with COVID-19, no sign was found of the virus or its direct cytopathic action, and they may be linked to the host immune response.
The authorization documents of all the available vaccines mentions the presence of dermatologic adverse effects and, after their clinical use, publications of various skin reactions continue to appear. As dermatologists, it is important to understand the characteristics of the different types of vaccine, their interaction with the host, and the range of systemic and dermatologic reactions that we may find.
SARS-CoV-2 VaccinesSARS-CoV-2 enters the host cell by binding to the angiotensin converting enzyme 2 (ACE2) by means of its surface spike (S) protein. The release of RNA leads to replication, transcription, and synthesis of viral RNA and the resulting structural proteins (viral RNA, nucleocapsid [N], membrane [M], envelope [E], and S) form the mature virion, which can escape the host cell to infect others.
In the host, SARS-CoV-2 induces an innate immune response, with the participation of IFN-I and other proinflammatory cytokines, and an adaptive immune response, in which T CD4+ and CD8+ lymphocytes react against the viral proteins S, M, and N, Nsp, and ORF3a. In the mucosa of the upper respiratory tract, contact with the virus induces the formation of secretory IgA. At the systemic level, IgG specifies against the S protein and the protein of its receptor-binding domain (RBD) is recruited from the serum to the lower respiratory tract. The responses of serum IgM and IgA are less intense1,6.
The vaccines train the immune system using harmless SARS-CoV-2 antigens to stimulate an immune response without causing disease. Following intramuscular administration, they essentially induce humoral immunity but are not able to improve the local response. Those administered intranasally induce a potentially sterilizing lgA-dependent local immunity but induce less effective systemic immunity. Some vaccines use adjuvants to activate the cell receptors and induce an innate immune response locally and in the regional lymph nodes, or to polarize the type of immune response desired. They improve the immune response but they also increase reactions to the vaccine6,7. Both inactivated-virus vaccines and protein-based viruses are formulated with adjuvants.
Each vaccine introduces one type of antigen, triggering a specific immune response that, in turn, generates the immune memory that makes it possible to combat SARS-CoV-2 in the event of future exposure. This antigen is processed by the antigen-presenting cells and is presented to the CD8+ and CD4+ T cells. Th1 cytokines also stimulate the CD8+ T cells and Th2 cytokines induce the production of neutralizing antibodies by the B cells.
All the vaccines induce some level of inflammation triggered by the activation of the innate immune response8. The imbalance of these responses may give rise to inflammatory reactions6, which can manifest in the skin.
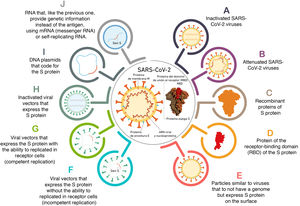
Anti-protein S antibodies inhibit the SARS-CoV-2–ACE-2 bond and the development of vaccines has thus focused on antigens derived from the S protein. The different forms used by the vaccines to introduce the antigen are shown in Fig. 1. And the different characteristics of the main vaccines available and under development are shown in Table 1.
Comparative characteristics of the different types of vaccine.
| Type of vaccine | Name/company/country | Composition | Special characteristics | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Inactivated SARS-CoV-2 viruses | CoronaVac® (Sinovac Biotech®, China) | SARS-CoV-2 cell culture and inactivation of the virus using chemical or physical methods (formaldehyde, heat, or ultraviolet light) | The immune response is aimed at S, M, E, and N proteins. | Safer than attenuated live vaccines; they cannot cause disease and generate fewer reactions. | They generate a weaker immune response. |
| Covaxin® (Bharat Biotech®, India) | They use adjuvants to potentiate their effect (aluminium hydroxide). | Local reactions to aluminium hydroxide | |||
| Intramuscular administration | |||||
| Viral vectors | Vaxzevria® (Oxford/AstraZeneca®, United Kingdom) | They use another virus, modified to express an antigen of SARS-CoV-2. | AstraZeneca uses a chimpanzee adenovirus designed to express S protein and modified to be unable to replicate in cells. | Conservation at normal refrigerator temperatures, which facilitates logistics and distribution in developing countries. | Risk of loss of efficacy if neutralized by pre-existing immunity. To prevent this, the vaccine uses vectors that are rare in humans or with low immunogenic ability. |
| Ad26Cov2-S (Janssen®, United States) | |||||
| Sputnik-V® (Gamaleya®, Russia) | Intramuscular administration | ||||
| Recombinant proteins | NVX-CoV2373 (Novavax®), United States | They use artificially created proteins of the virus, such as the receptor-binding domain (RBD) of the S protein. | Obtained through genetic engineering, supported by experience with other authorized vaccines (hepatitis B, HPV, influenza) | Manufacturing is simpler | They generate a weaker immune response. |
| They use adjuvants to potentiate their effect (MATRIX-M). | |||||
| Intramuscular administration | |||||
| RNA vaccines | BNT162/Comirnaty® (Pfizer®/BioNTech®, Germany, United States) | They contain messenger RNA (mRNA) that teaches the cells to produce the protein that triggers an immune response to infection (it induces ribosomal production of proteins that code for the antigen). | More frequent adverse reactions (mRNA is a potent immunity activator) | They are not able to produce disease (like attenuated-virus vaccines) | Instability |
| mRNA 1273 (Moderna®, United States) | Self-replicating RNA vaccines contain an RNA-polymerase complex that amplifies the mRNA and produces a larger amount of antigen. | Particular precaution in people with a history of allergies and anaphylaxis | They are not able to form part of the host genome (like DNA vaccines) | Difficult conservation conditions | |
| Intramuscular administration | They generate a stronger and longer-lasting immune response. | Ability to stimulate the innate immune system, with production of INF1 and eventual reduction of efficacy32,33 | |||
| They stimulate the innate immune system. mRNA and lipid carriers act as an adjuvant. |
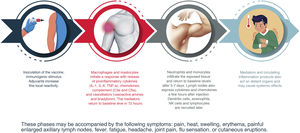
Reactogenicity or the physical manifestation of the inflammatory response may present as a local reaction at the injection site (pain, heat, reddening, swelling, hardness) and/or with systemic symptoms (fever, myalgia, headache, etc.). The sequence of reactions induced from the moment of contact with the vaccine are shown in Fig. 2.
The tolerability and immunogenicity profile of the vaccines varies depending on the type of antigen and the adjuvant used. RNA vaccines (potent activator of the innate immune response) induce frequent, though mild, adverse reactions. Other factors that modify these effects are age (more reactogenicity in young people), a high body mass index (perhaps linked to a poor subcutaneous injection technique), pre-existing immunity, the route and site of administration, the injection technique, sex (more reactogenicity in women), some genetic and racial factors, and anxiety.
Adverse Effects of the COVID-19 VaccinesThe clinical trials prior to approval of the available vaccines notified adverse effects, including skin reactions, sometimes with few details, which are summarized in Table 29–12.
Adverse effects described in clinical trials.
| Systemic |
|---|
| Very frequent: headache, fatigue, and joint pain |
| Less frequent: fever, enlarged lymph nodes, nausea/vomiting, diarrhea, and chills |
| With the Moderna® and Pfizer® vaccines, they are more frequent after the second dose and with the AstraZeneca® vaccine, they are more frequent after the first dose. |
| Local |
| Extremely frequent: pain and sensitivity at the injection site (84.2% of participants after the first dose) |
| Less frequent: erythema and edema11 |
| Local inflammation greater than 2.5 cm was considered an adverse effect and greater than 10 cm was considered severe. |
| Delayed local reactions (a week or more after the injection) 0.8% of participants after the first dose; 0.2% after the second dose. (Moderna®) |
| Other cutaneous adverse effects |
| Rosacea and cellulitis (AstraZeneca®) |
| Urticaria (1), maculopapular rash (1), non-anaphylactic hypersensitivity reactions (1.5% of those vaccinated) and edema at sites where dermal filler had been used (3) (Moderna®) |
| Non-anaphylactic hypersensitivity reactions in 0.63% (Pfizer®) |
| Urticaria (5) and urticaria with angioedema (1), 7 and 4 days after receiving the vaccine (Janssen®) |
Cutaneous adverse effects published after the vaccine reached the market are, partly, the same as those described in the clinical trials. These include the following:
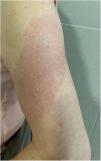
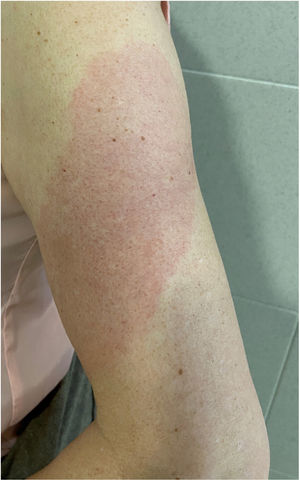
COVID-Arm (Fig. 3)This is the most commonly reported reaction. It involves the appearance of erythematous, edematous plaques of different sizes in the area where the vaccine was administered. These eruptions have been described with other vaccines, such as the combined pneumococcal vaccine13.
The first published cases8 appeared between 7 and 10 days after the first dose of the Moderna® vaccine, which was initially attributed to a hypersensitive reaction to the polyethylene glycol contained in the vaccine. In another series, however, the intradermal test with polyethylene glycol-polysorbate was only positive in one patient with a reaction that appeared only after the second dose. This does not support an IgE-mediated reaction to these compounds as a cause14. Another study supports a delayed or T-cell mediated hypersensitivity reaction as a cause, based on the histopathology that showed the presence of superficial perivascular and perifollicular lymphocytic infiltrates with mastocytes and disperse eosinophils15,16. A retrospective study analyzed the cutaneous reactions in a group of health workers vaccinated with the Pfizer® vaccine. A delayed local reaction was observed in 2.1%, after both the first and second dose. As reported in other series, in half of cases, the reaction was repeated after the second dose. Two were biopsied and a superficial and deep perivascular lymphocyte infiltrate was found, with dilated vessels, intraluminal neutrophils, and immune staining negative for the SARS-CoV-2 spike protein 1A917.
All reported cases resolved with conservative treatment, such as local application of cold, topical corticosteroids, and/or antihistamines. No severe adverse reactions have been reported following the second dose and changing the guidelines is therefore not recommended.
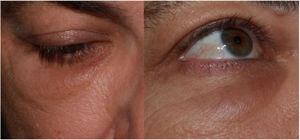
Inflammatory Reactions in Areas With Hyaluronic Acid Dermal Filler (Fig. 4)The appearance of erythema, edema, and nodules in areas that had previously been subjected to dermal fillers following processes such as viral infections or vaccines is well known18, and this has also been described in coincidence with SARS-CoV-2 infection and in some isolated cases after vaccination against COVID-1919,20. Taking into account the number of vaccines administered globally and the number of people who have dermal fillers, it seems likely that the frequency is very low21.
A suggested pathophysiological mechanism is a cascade of events that begins with deficient phagocytosis of the hyaluronic acid, formation of a biofilm around the filler material, activation of T cells, and formation of fibrosis and granulomas. These alterations favor the presence of Th1/CD8+ T cells and the high concentrations of ACE2 in the resident cells. This would lead to abnormal regulation between the production of proinflammatory angiotensin II and anti-inflammatory angiotensin I. The binding of the S protein to ACE2, induced by the vaccine, would tilt the balance toward inflammation. In 5 cases, this hypothesis justified treatment of these reactions by blocking the angiotensin II with an ACE inhibitor, with good results. The authors recommend lisinopril 5 mg/d, doubling the dosage if no improvement is achieved in 72 h and pretreatment before the second dose of the vaccine of patients who presented this complication after the first dose.
It is important to bear in mind that, although there are currently few cases, all those reported have evolved favorably regardless of the prescribed treatment, which has been highly diverse. Cases have been reported that were treated with systemic antihistamines, antibiotics, or corticosteroids, intralesional corticosteroids and 5-fluorouracil, and injections of hyaluronidase. A more thorough understanding and broader experience are doubtless necessary before reaching conclusions.
Other Cutaneous EruptionsAs with COVID-19, a wide variety of cutaneous manifestations have been reported coinciding with vaccination and with no other apparent cause. Morbilliform eruptions15, urticarial eruptions17, maculopapular eruptions22, lichen planus23,24, erythema multiforme25, and reactivations of herpes zoster26. These data corroborate the recent publication of 414 reactions following the Moderna® and Pfizer® vaccines reported by different health care professionals through the register of SARS-CoV-2 cutaneous manifestations of the American Academy of Dermatology. No severe reactions were found and half of patients with reactions following the first dose did not present these reactions after the second dose. Different reactions have been added, which were observed both after the first dose and after the second dose, such as erythromelalgia and perniosis27.
Other ManifestationsSelf-limiting enlarged supraclavicular lymph nodes have been reported and have been linked to the injection at an inappropriate site, higher than recommended (2–3 fingers below the acromion)28 and rare anaphylactic reactions with no fatalities29 but in a higher percentage than with the usual vaccines (4.2 cases per million compared to 1 case per million).
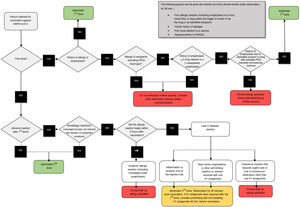
The CDC has issued recommendations for mRNA vaccines, about when to give a green light, take precautions, or avoid administering them, which are summarized in Fig. 5. Although most patients who have had anaphylactic reactions have a history of allergies30, atopic patients with rash and with eczema, while they should be correctly treated at the moment of vaccination, should not delay vaccination, and do not have an increased risk of severe allergic reactions31.
COVID-19 Vaccines-Skin StudyAs occurred with the COVID-19 emergency, the mass administration to the population of a newly created vaccine and technology is bringing to light a wide variety of cutaneous manifestations. As dermatologists, it is our mission to be watchful, to analyze, categorize, evaluate the significance and the consequences of each manifestation, and communicate our findings to the pharmacovigilance bodies and to the scientific community.
With this goal and with the support of the Spanish Academy of Dermatology, may dermatologists in Spain have proposed recording as many dermatologic adverse effects as possible.
At present, most of the cases reported correspond to women and to the Pfizer vaccine®. A similar number of effects has been reported after the first dose and after the second dose. The most frequent manifestations are covid-arm, urticarial eruptions, and reactivation of herpes zoster34. Information: vacunascovidpiel@gmail.com; https://aedv.es/reacciones-cutaneas-tras-la-administracion-de-vacunas-frente-a-sars-cov-2/
ConclusionThe vaccines have a proven safety profile. Nevertheless, they do present systemic and local reactions that we should be aware of.
The correct categorization of cutaneous reactions to the vaccines will make it possible to provide adequate information to our patients, prevent unnecessary rejection of vaccination, reduce severe adverse effects in predisposed individuals, and gain a deeper understanding of the pathophysiological mechanisms underlying the immune reaction to SARS-CoV-2.
FundingThis study has not received funding of any kind.
Conflicts of interestThe authors declare that they have no conflicts of interest.
The authors would like to thank the Spanish Academy of Dermatology for its support, Álvaro Frías Sánchez for the graphics, and José Luis Martínez Amo for allowing the use of Fig. 4.
Please cite this article as: Galván-Casas C, Català A, Muñoz-Santos C. Vacunas frente a SARS-CoV-2 y piel. Actas Dermosifiliogr. 2021;112:828–836.