It is necessary to expand the knowledge in the use of apremilast in clinical practice. The APPRECIATE study (NCT02740218) aims to describe the characteristics of patients with psoriasis treated with apremilast, to evaluate their perspectives and those of dermatologists, as well as the outcomes obtained in clinical practice in Spain.
MethodsObservational, retrospective, cross-sectional, multicenter study of patients with chronic plaque psoriasis who could be contacted 6 (±1) months after apremilast initiation. The data were obtained from medical records and questionnaires from patients and physicians.
ResultsA total of 80 patients were evaluated; at apremilast onset, they showed mean (standard deviation, SD) Psoriasis Area and Severity Index (PASI) = 8.3 (5.3), mean (SD) Dermatology Life Quality Index (DLQI) = 8.9 (6.6). At six months, 58.8% (n = 47) of patients continued apremilast treatment (discontinuations due to lack of efficacy [16.3%], safety/tolerability [20.0%]). In patients continuing treatment, PASI75 was achieved by 36.7% of patients; mean (95% CI) DLQI score was 2.2 (0.7–3.6) and mean (SD) Patient Benefit Index score was 2.8 (0.8). Compliance with physicians’ expectations was correlated with benefits reported by patients (r = 0.636). Adverse events were reported by 56.3% of patients (the most common were diarrhoea and nausea).
ConclusionsPatients receiving apremilast for 6 months in Spanish clinical practice, reported substantial improvements in their quality of life (mean DLQI reduced by more than 6 points) and disease severity (PASI75 achieved by over one-third of patients), despite less skin involvement than patients who enrolled in clinical trials.
Es conveniente ampliar el conocimiento del manejo de apremilast en práctica clínica. El estudio APPRECIATE (NCT02740218) pretende describir las características de pacientes con psoriasis tratados con apremilast, evaluar sus perspectivas y las de sus dermatólogos, y los resultados obtenidos en la práctica clínica española.
MétodosEstudio observacional, retrospectivo, transversal y multicéntrico en pacientes con psoriasis crónica en placas, a los que se visitó seis (±1) meses después de iniciar apremilast. Los datos se obtuvieron de las historias clínicas y cuestionarios realizados por pacientes y dermatólogos.
ResultadosSe evaluaron 80 pacientes, al iniciar apremilast presentaban Psoriasis Area and Severity Index (PASI) medio (desviación estándar, DE) = 8,3 (5,3) y Dermatology Life Quality Index (DLQI) medio (DE) = 8,9 (6,6). A los seis meses, el 58,8% (n = 47) continuaba con apremilast (discontinuaciones: falta de eficacia [16,3%], seguridad/tolerabilidad [20,0%]). En pacientes que continuaban en tratamiento, el PASI75 fue alcanzado por el 36,7%; la puntuación DLQI media (IC 95%) fue 2,2 (0,7–3,6) y Patient Benefit Index medio (DE) 2,8 (0,8). El cumplimiento de las expectativas de los dermatólogos se correlacionó con los beneficios descritos por los pacientes (r = 0,636). El 56,3% reportó acontecimientos adversos (diarrea y náuseas los más frecuentes).
ConclusionesLos pacientes que recibieron apremilast durante seis meses en la práctica clínica en España reportaron una mejoría en su calidad de vida (DLQI medio se redujo más de seis puntos) y en la gravedad de la enfermedad (PASI75 alcanzado por más de un tercio de los pacientes), a pesar de presentar una afectación cutánea menor que aquellos pacientes incluidos en ensayos clínicos.
Apremilast (an oral phosphodiesterase-4 inhibitor) was approved in the European Union for the treatment of moderate to severe chronic plaque psoriasis and psoriatic arthritis in 2015. In clinical trials, apremilast demonstrated its efficacy and safety in patients with moderate to severe psoriasis previously treated with systemic therapies (ESTEEM 1 and 2)2,3, with no prior biologic therapy (LIBERATE)4, and with no previous biologic or systemic therapy (UNVEIL). Subsequently, the effectiveness shown in series in clinical practice, in different patient populations to those enrolled in the pivotal clinical trials, appeared to be greater. It therefore seemed appropriate to extent our knowledge of the use and effectiveness of apremilast in a clinical practice setting, compared with the aforementioned pivotal trials, and generate data on patient and physician satisfaction with treatment.
The objective of the APPRECIATE study (NCT02740218) was to describe the characteristics of patients treated with apremilast in routine clinical practice and assess the patient and dermatologist perspectives on initiation of treatment, along with the outcomes and clinical benefit obtained. The results from Germany, Austria, Ireland, United Kingdom, Sweden, and Switzerland were published recently. This article presents the results for Spain.
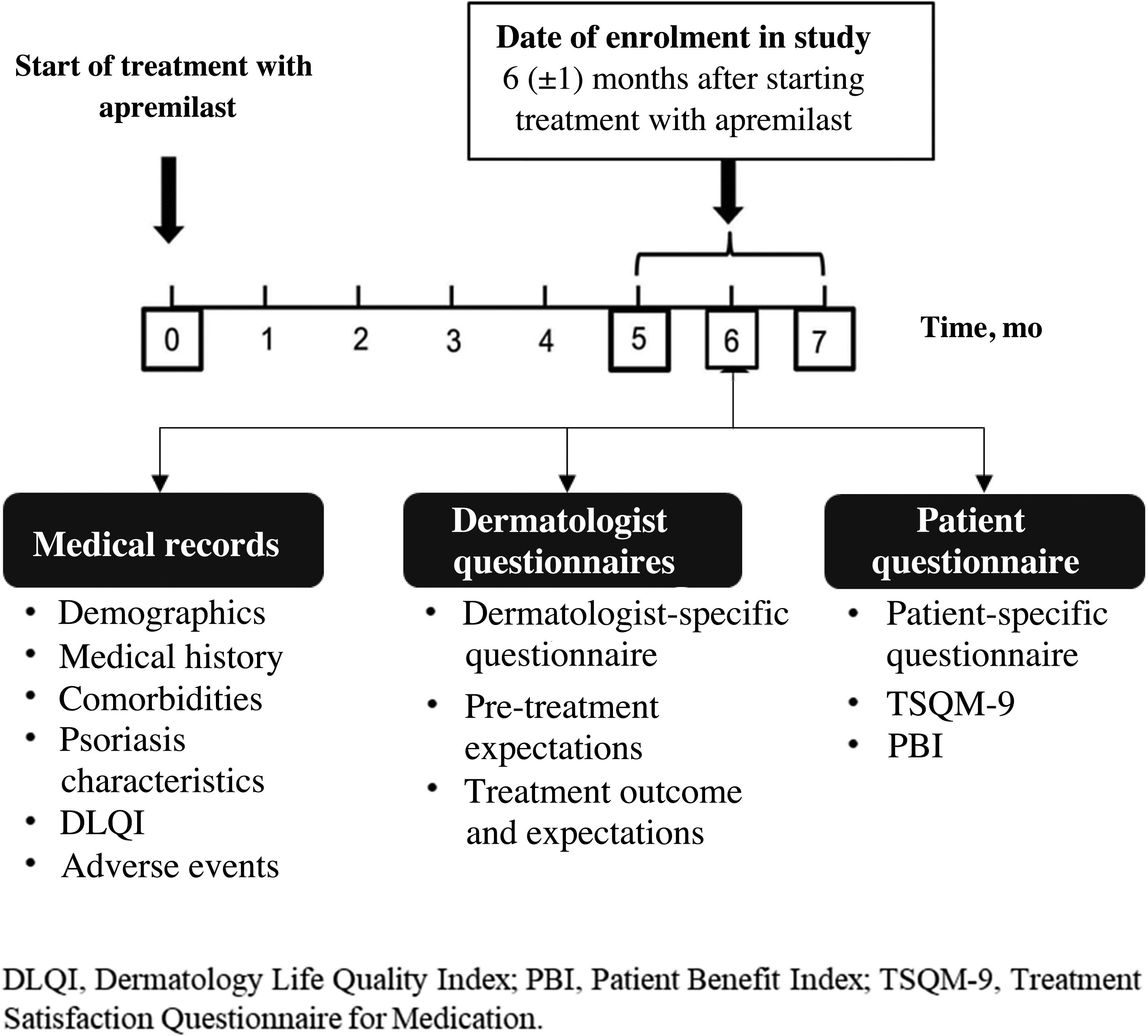
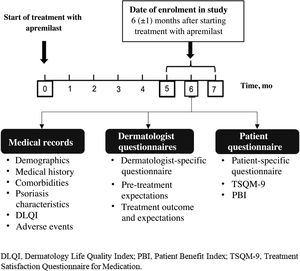
MethodsStudy DesignAPPRECIATE is an international, observational, retrospective, cross-sectional study that collected outcomes 6 months after starting apremilast treatment in everyday clinical practice (Fig. 1). Here, we present the analyses of data collected in 13 Spanish hospitals, where patient recruitment began in 2018 and ended in November 2019.
Study PopulationEligible patients were adults (≥18 years) diagnosed with chronic plaque psoriasis who had initiated treatment with apremilast 6 ± 1 months prior to enrolment in the study. All consecutive patients who met the above inclusion criteria were invited to participate, regardless of whether they had discontinued apremilast at the time of assessment. Inclusion in the study did not interfere in any way with the routine clinical practice of the participating dermatologists. The only exclusion criterion was the participation of patients in a clinical trial. The study was conducted according to the Declaration of Helsinki and was approved by the ethics committees of the participating centers. All participants signed the informed consent before any data were recorded.
Data CollectionThe study involved a single face-to-face visit at 6 ± 1 months after starting apremilast treatment. At this visit, data from patient medical records were collected and the questionnaires were completed by the dermatologists and patients.
The following data were recorded from the patient medical records: demographic data, comorbidities, disease severity (Psoriasis Area and Severity Index [PASI] and Body Surface Area [BSA])7, clinical manifestations (pruritus, fatigue, nails, scalp, palmoplantar, inverse genital and nongenital psoriasis, palmoplantar pustulosis, and psoriatic arthritis), state of treatment with apremilast (date and reason for discontinuation if applicable), prior psoriasis treatments, quality of life (QoL) measured with the Dermatology Quality of Life Index (DQLI), and adverse events (AEs).
The dermatologists completed 3 study-specific satisfaction questionnaires, which collected information on reasons for using apremilast (1), the effects of treatment on symptoms, treatment tolerability, and effectiveness of apremilast on specific psoriasis manifestations (2), and meeting expectations and general success (3).
The patients also completed 3 questionnaires: Patient Benefit Index (PBI)9, Treatment Satisfaction Questionnaire for Medication (TSQM-9), and the study-specific questionnaire, which collected demographic and socioeconomic data, lifestyle factors, and expectations and experience with apremilast.
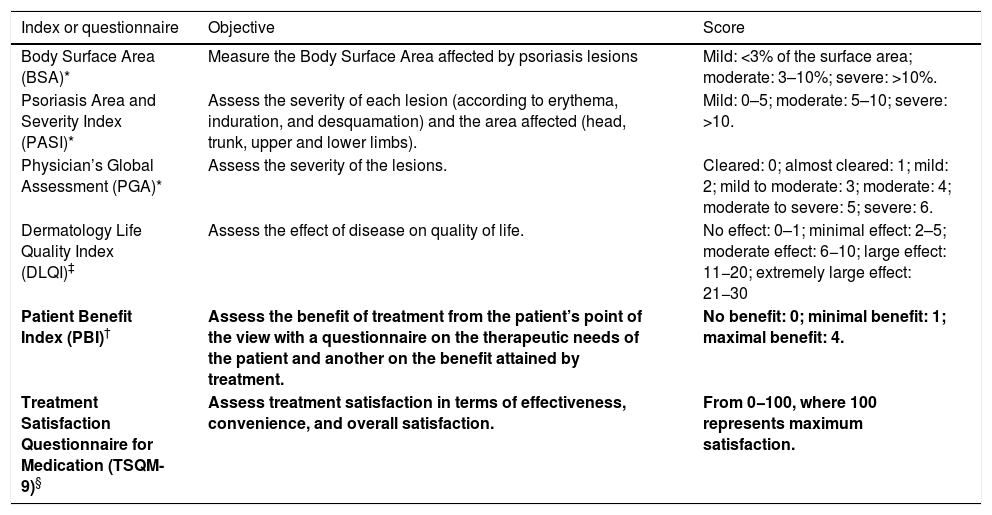
Table 1 summarizes the main outcome measures.
Main tools for outcome measures.
| Index or questionnaire | Objective | Score |
|---|---|---|
| Body Surface Area (BSA)* | Measure the Body Surface Area affected by psoriasis lesions | Mild: <3% of the surface area; moderate: 3–10%; severe: >10%. |
| Psoriasis Area and Severity Index (PASI)* | Assess the severity of each lesion (according to erythema, induration, and desquamation) and the area affected (head, trunk, upper and lower limbs). | Mild: 0–5; moderate: 5–10; severe: >10. |
| Physician’s Global Assessment (PGA)* | Assess the severity of the lesions. | Cleared: 0; almost cleared: 1; mild: 2; mild to moderate: 3; moderate: 4; moderate to severe: 5; severe: 6. |
| Dermatology Life Quality Index (DLQI)‡ | Assess the effect of disease on quality of life. | No effect: 0–1; minimal effect: 2–5; moderate effect: 6−10; large effect: 11−20; extremely large effect: 21−30 |
| Patient Benefit Index (PBI)† | Assess the benefit of treatment from the patient’s point of the view with a questionnaire on the therapeutic needs of the patient and another on the benefit attained by treatment. | No benefit: 0; minimal benefit: 1; maximal benefit: 4. |
| Treatment Satisfaction Questionnaire for Medication (TSQM-9)§ | Assess treatment satisfaction in terms of effectiveness, convenience, and overall satisfaction. | From 0−100, where 100 represents maximum satisfaction. |
Demographic data, disease characteristics, outcomes of treatment, and AEs were analyzed with descriptive statistics. The 95% confidence intervals (CI) were calculated. Missing values were not imputed. Results were stratified by status of apremilast therapy (ongoing versus discontinued). Correlations with outcome measures were calculated. The statistical analyses were performed with the SAS program version 9.4.
ResultsPopulation CharacteristicsIn Spain, 86 patients were invited to participate in the APPRECIATE study, of whom 82 were enrolled. Finally, 80 patients were included in the safety population and full analysis set (FAS); 2 patients (2.4%) were excluded because they had not been diagnosed with psoriasis.
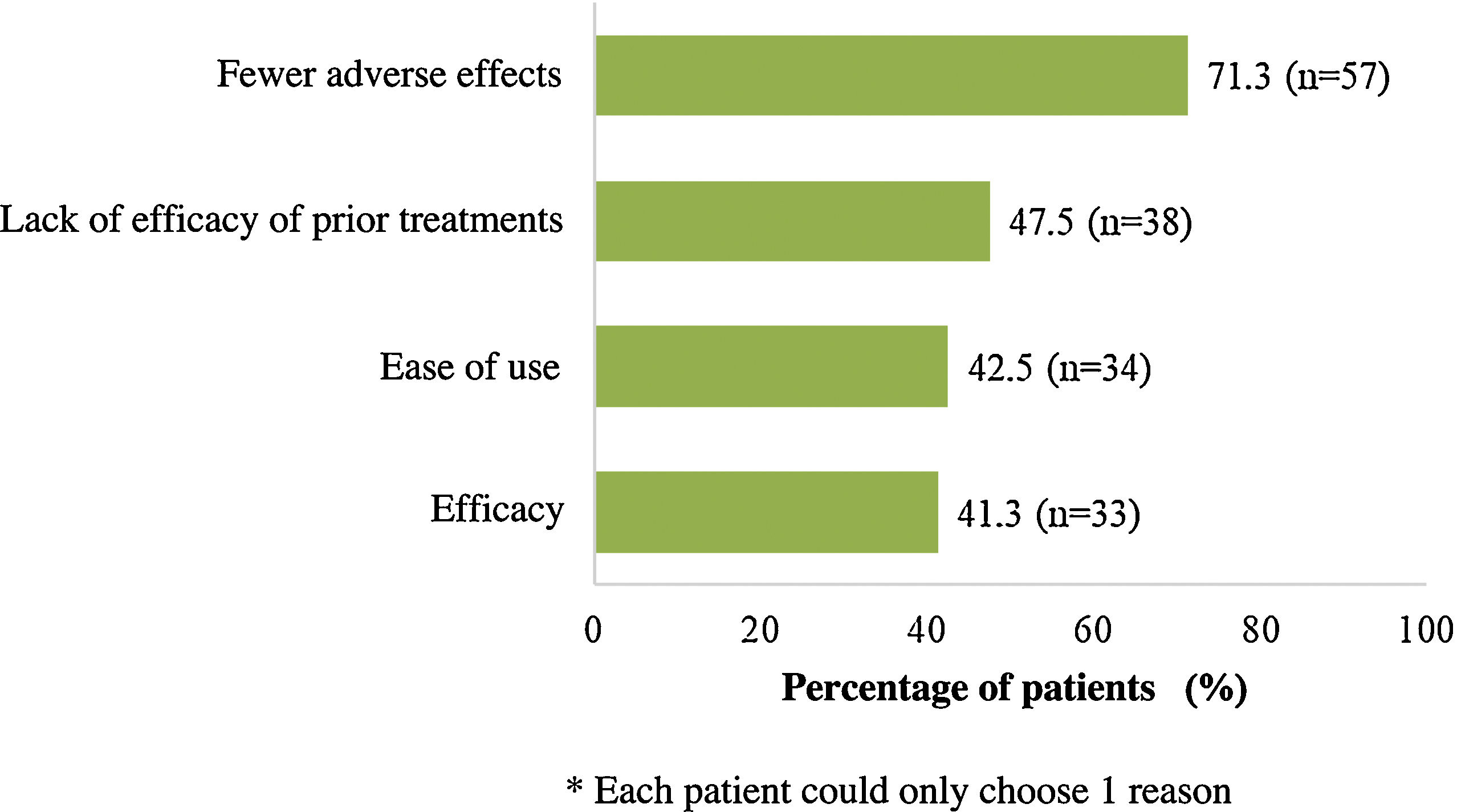
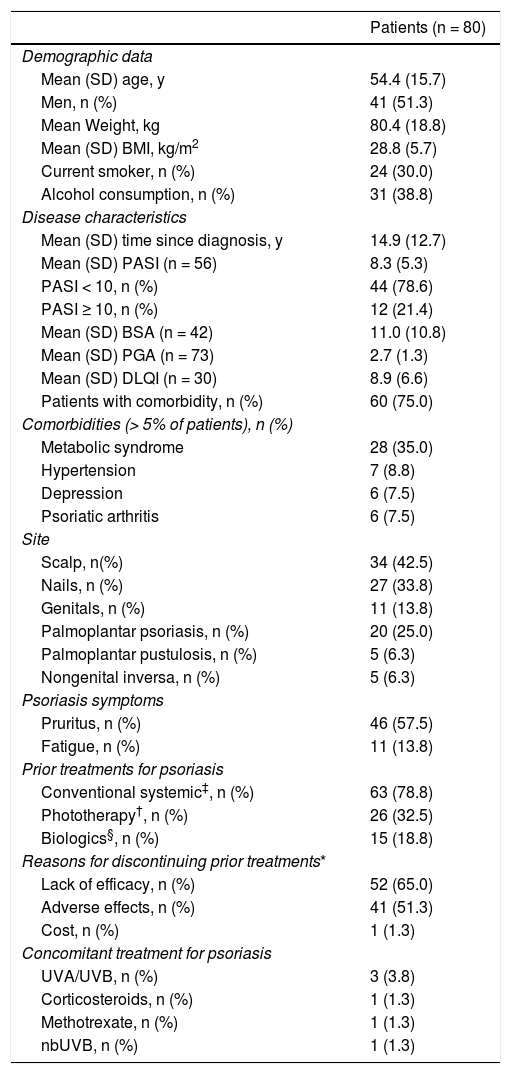
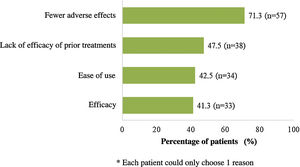
At the time of starting treatment with apremilast, patients had a mean (SD) baseline score for PASI and BSA of 8.3 (5.3) and 11.0 (10.8), respectively. In addition, the majority of patients (78.6%) had a PASI score <10 (mild to moderate intensity) and the mean (SD) DLQI score was 8.9 (6.6), indicating a moderate impact on QoL (Table 2). The main symptom was pruritus (57.5%) and the most frequent site was the scalp, followed by the nails (42.5% and 33.8%, respectively). Overall, 75.0% of patients (n = 60) had associated comorbidities (Table 2). At the start of treatment with apremilast, 16.3% (n = 13) and 11.3% (n = 9) of patients received treatment for anxiety and depression, respectively. Most patients (n = 70, 87.5%) had received at least 1 prior systemic treatment for psoriasis (1 treatment in 26 patients [32.5%], 2 treatments or more in 19 [23.8%]) and 6 (7.5%) continued with some other concomitant systemic treatment (Table 2). Fig. 2 shows the main reasons for considering apremilast treatment.
Demographic data and disease characteristics at start of treatment with apremilast.
| Patients (n = 80) | |
|---|---|
| Demographic data | |
| Mean (SD) age, y | 54.4 (15.7) |
| Men, n (%) | 41 (51.3) |
| Mean Weight, kg | 80.4 (18.8) |
| Mean (SD) BMI, kg/m2 | 28.8 (5.7) |
| Current smoker, n (%) | 24 (30.0) |
| Alcohol consumption, n (%) | 31 (38.8) |
| Disease characteristics | |
| Mean (SD) time since diagnosis, y | 14.9 (12.7) |
| Mean (SD) PASI (n = 56) | 8.3 (5.3) |
| PASI < 10, n (%) | 44 (78.6) |
| PASI ≥ 10, n (%) | 12 (21.4) |
| Mean (SD) BSA (n = 42) | 11.0 (10.8) |
| Mean (SD) PGA (n = 73) | 2.7 (1.3) |
| Mean (SD) DLQI (n = 30) | 8.9 (6.6) |
| Patients with comorbidity, n (%) | 60 (75.0) |
| Comorbidities (> 5% of patients), n (%) | |
| Metabolic syndrome | 28 (35.0) |
| Hypertension | 7 (8.8) |
| Depression | 6 (7.5) |
| Psoriatic arthritis | 6 (7.5) |
| Site | |
| Scalp, n(%) | 34 (42.5) |
| Nails, n (%) | 27 (33.8) |
| Genitals, n (%) | 11 (13.8) |
| Palmoplantar psoriasis, n (%) | 20 (25.0) |
| Palmoplantar pustulosis, n (%) | 5 (6.3) |
| Nongenital inversa, n (%) | 5 (6.3) |
| Psoriasis symptoms | |
| Pruritus, n (%) | 46 (57.5) |
| Fatigue, n (%) | 11 (13.8) |
| Prior treatments for psoriasis | |
| Conventional systemic‡, n (%) | 63 (78.8) |
| Phototherapy†, n (%) | 26 (32.5) |
| Biologics§, n (%) | 15 (18.8) |
| Reasons for discontinuing prior treatments* | |
| Lack of efficacy, n (%) | 52 (65.0) |
| Adverse effects, n (%) | 41 (51.3) |
| Cost, n (%) | 1 (1.3) |
| Concomitant treatment for psoriasis | |
| UVA/UVB, n (%) | 3 (3.8) |
| Corticosteroids, n (%) | 1 (1.3) |
| Methotrexate, n (%) | 1 (1.3) |
| nbUVB, n (%) | 1 (1.3) |
Abbreviations: BMI, body mass index; BSA, Body Surface Area; DLQI, Dermatology Life Quality Index; nbUVB, narrow band UVB; PASI, Psoriasis Area and Severity Index; PGA, Physician’s Global Assessment.
Conventional systemic treatment included methotrexate (n = 38; 47.5%), acitretin (n = 25; 31.3%), retinoids (n = 16; 20.0%), cyclosporin (n = 14; 17.5%), alitretinoin (n = 2; 2.5%), fumaric acid (n = 2; 2.5%), sulfasalazine (n = 1; 1.3%).
Overall, 58.8% of patients analyzed (n = 47) had ongoing apremilast treatment after 6 months. The patients who discontinued the drug had used it for a mean (SD) of 103.5 (64.3) days. The main reasons for discontinuation were lack of efficacy (13/18 patients [16.3%]) and safety/tolerability reasons (16/80; 20.0%).
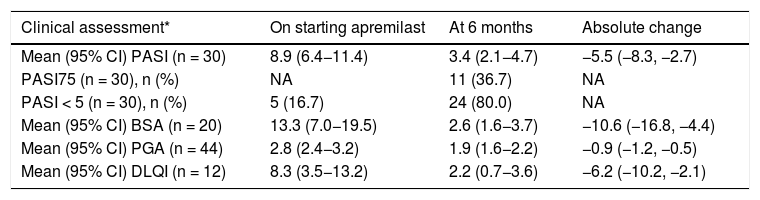
In patients who continued with apremilast after 6 months and for whom values for the various outcome measures were available at the start of treatment and at the follow-up visit, the mean PASI score at follow-up (30/47 patients [63.8%]) was 3.4 (95% CI: 2.1–4.7), corresponding to an improvement of 54.8% (Table 3). The PASI75 goal was reached by 36.7% of patients (11/30), and 80.0% (24/30) achieved PASI < 5 (Table 3). Of the patients who had a PASI < 10 at the start of treatment (n = 23), 26.1% (6/23; 95% CI: 10.2%–48.4%) achieved PASI75 and 78.3% (18/23) achieved PASI < 5; among the patients with PASI ≥ 10 on starting treatment (n = 7), 5 achieved PASI75 and 5 PASI < 5. The mean DLQI score at the follow-up visit (12/47; 25.5%) was 2.2 (95% CI: 0.7–3.6), which represents a mean improvement of 6.2 points (Table 3). The 7 patients with DLQI ≥ 4 at the start of treatment achieved an improvement of ≥4 points at 6 months.
Change in clinical assessments.
| Clinical assessment* | On starting apremilast | At 6 months | Absolute change |
|---|---|---|---|
| Mean (95% CI) PASI (n = 30) | 8.9 (6.4−11.4) | 3.4 (2.1−4.7) | −5.5 (−8.3, −2.7) |
| PASI75 (n = 30), n (%) | NA | 11 (36.7) | NA |
| PASI < 5 (n = 30), n (%) | 5 (16.7) | 24 (80.0) | NA |
| Mean (95% CI) BSA (n = 20) | 13.3 (7.0−19.5) | 2.6 (1.6−3.7) | −10.6 (−16.8, −4.4) |
| Mean (95% CI) PGA (n = 44) | 2.8 (2.4−3.2) | 1.9 (1.6−2.2) | −0.9 (−1.2, −0.5) |
| Mean (95% CI) DLQI (n = 12) | 8.3 (3.5−13.2) | 2.2 (0.7−3.6) | −6.2 (−10.2, −2.1) |
Abbreviations: BSA, Body Surface Area; CI, confidence interval; DLQI, Dermatology Life Quality Index; NA, not applicable; PASI, Psoriasis Area and Severity Index; PGA, Physician’s Global Assessment.
According to the dermatologist evaluation, 73.8% of the patients (n = 59) had an improvement in the general assessment of clearance of psoriasis, 61.3% (n = 49) had an improvement in overall wellbeing, and 56.3% (n = 45) achieved normal everyday life. Among the patients who showed signs and manifestations specific to psoriasis, the dermatologists confirmed that 80.4% (37/46) showed an improvement in pruritus, 67.6% (23/34) in scalp manifestations, 63.0% (17/27) in nail involvement, and 55.0% (11/20) in palmoplantar lesions. The dermatologists indicated that the results exceeded their expectations in half of the patients treated (50.0%; n = 40).
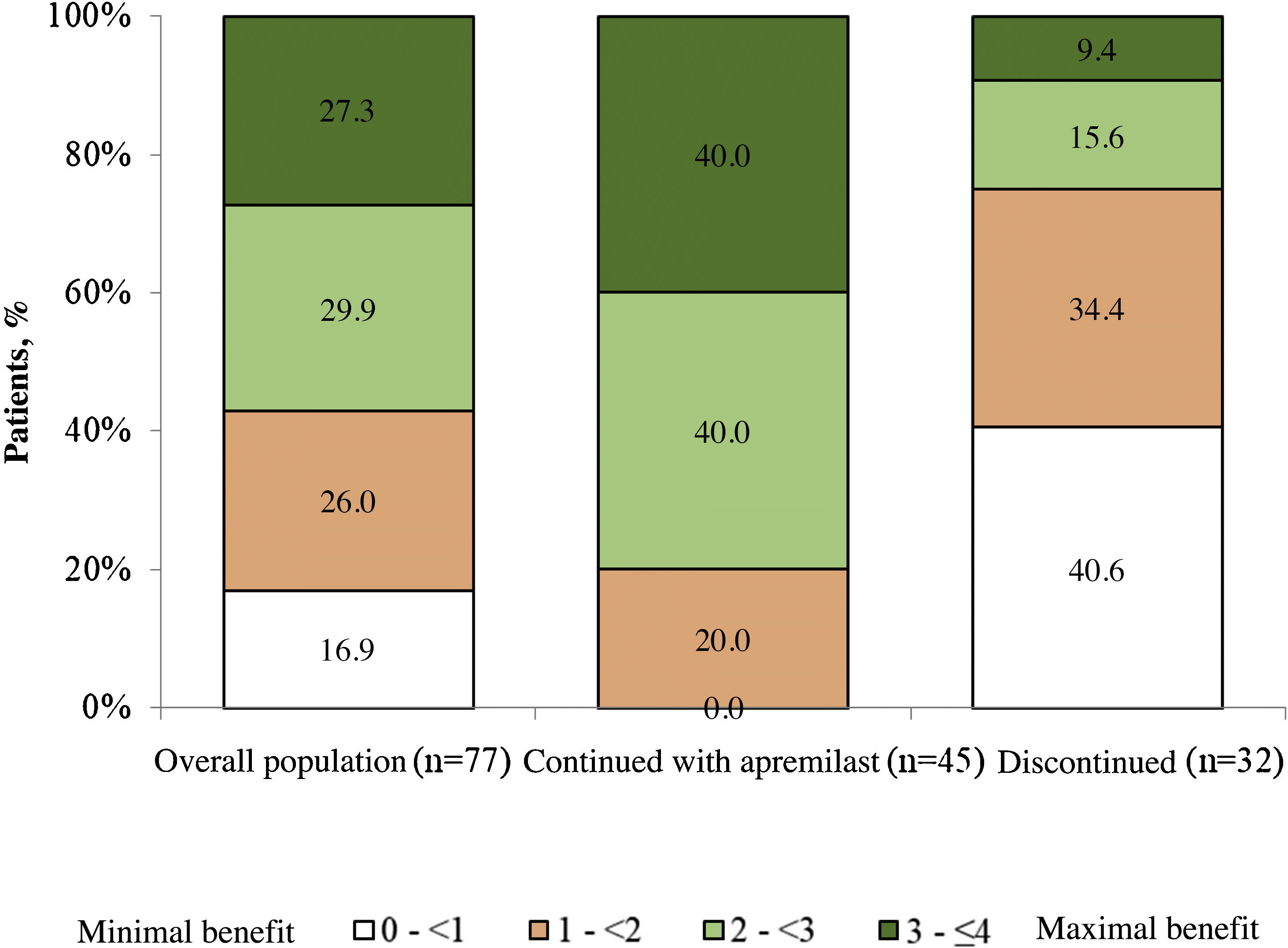
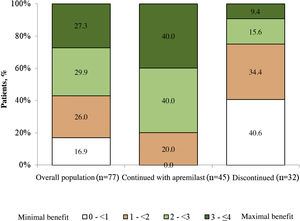
Patient satisfaction with apremilastThe mean (SD) PBI score for the overall population was 2.2 (1.2) and 83.2% of the patients (n = 64) achieved PBI ≥ 1 (significant clinical benefit) (Fig. 3). In patients who continued with apremilast treatment, the mean (SD) PBI score was 2.8 (0.8) and 100.0% (n = 45) achieved PBI ≥ 1 (Fig. 3).
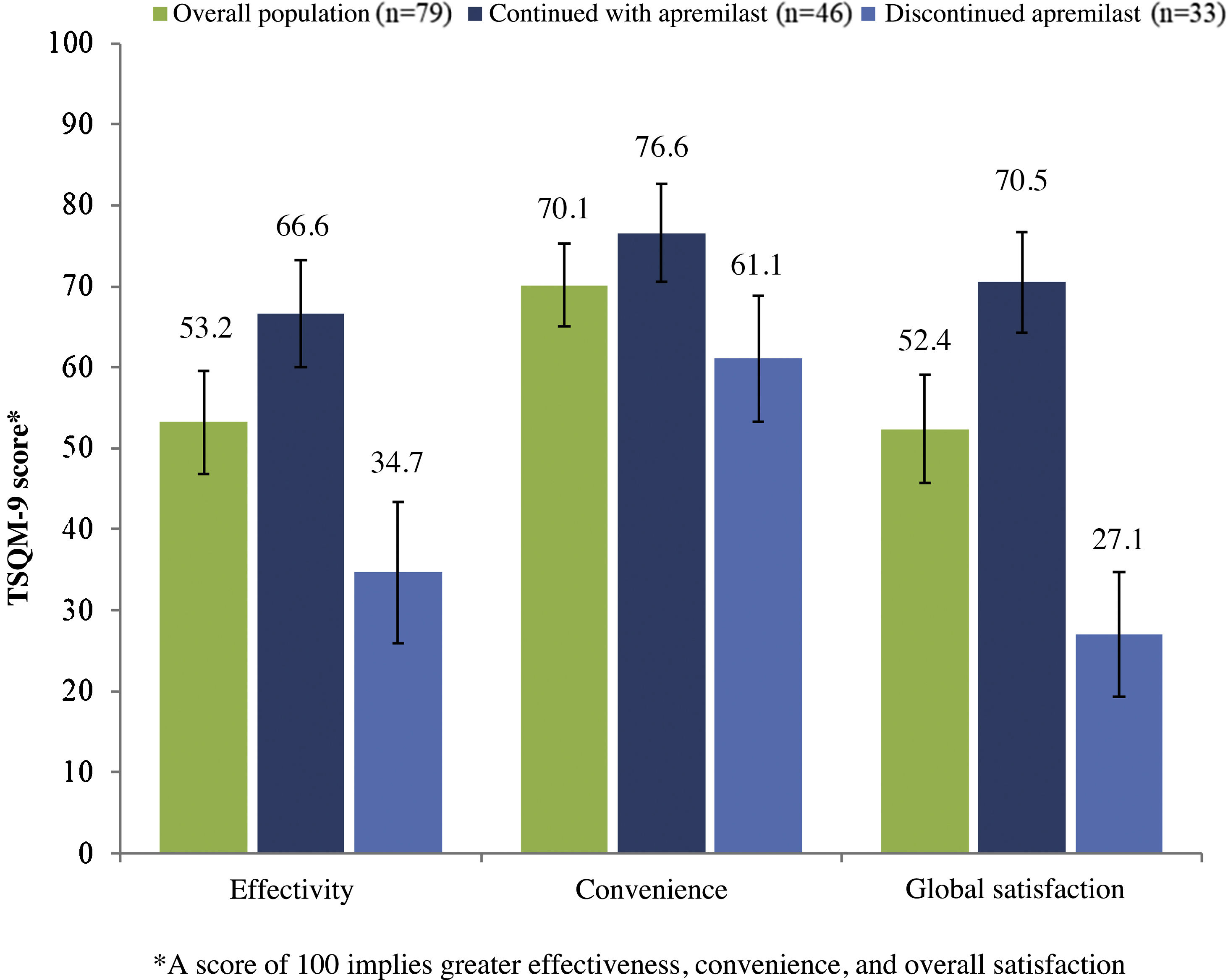
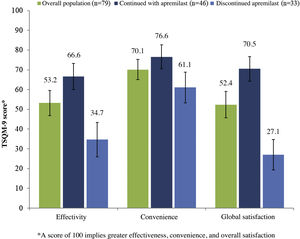
In patients who continued with apremilast, the overall mean (SD) score for satisfaction with treatment (TSQM-9) was 66.6 (22.9), 76.6 (21.0), and 70.5 (21.5) for the effectiveness, convenience, and overall satisfaction subscales, respectively (Fig. 4). In this same group of patients, a reduction in psoriasis symptoms was observed during the first month of treatment in 48.9% (n = 23), and 59.6% (n = 28) considered that apremilast enabled them to live a normal life.
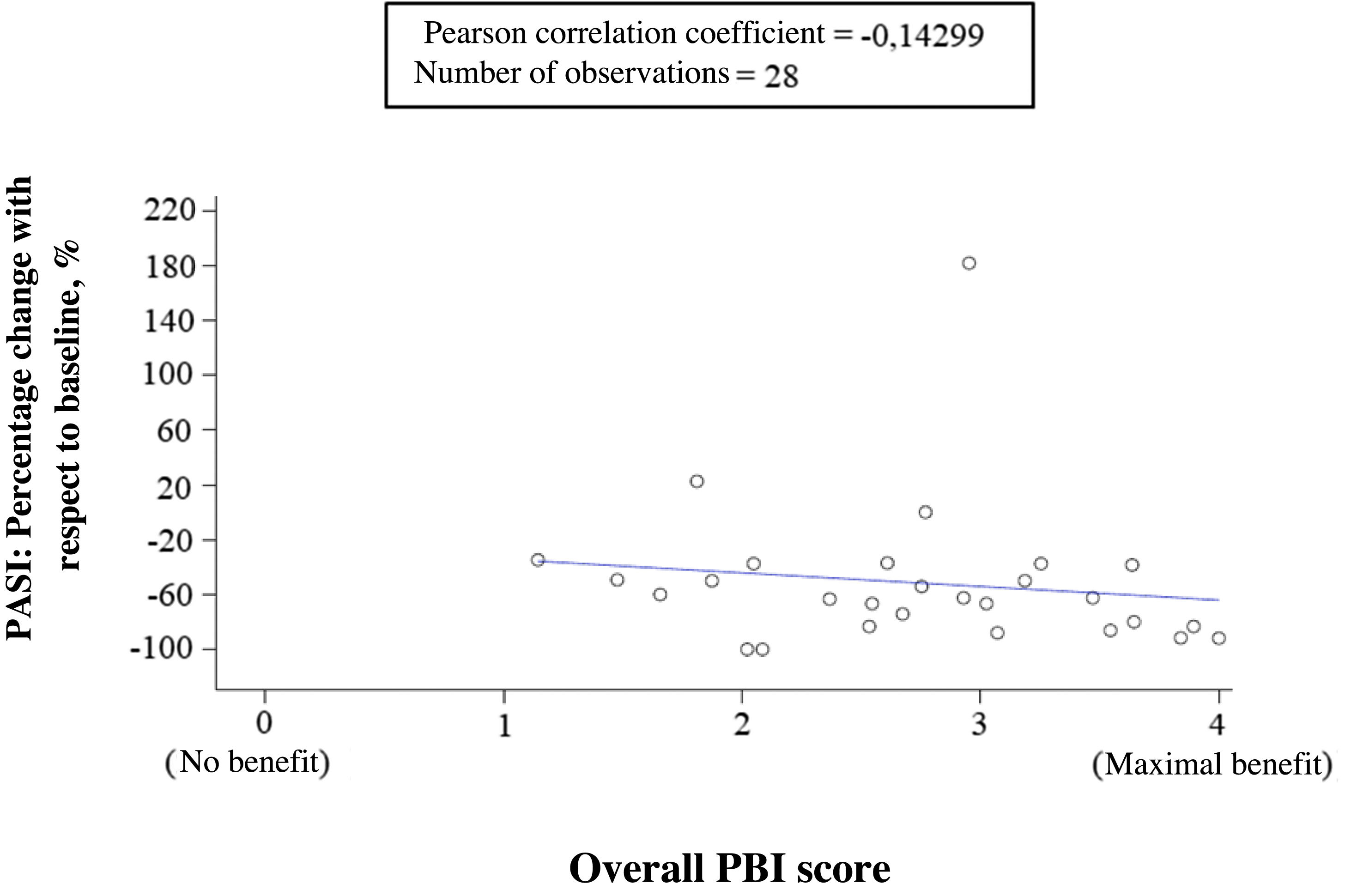
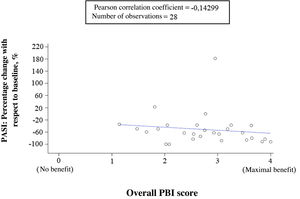
Correlation between clinical outcomes, expectations, and benefits reported by dermatologists and patientsThe correlation between percentage change from baseline in PASI and treatment benefit (overall PBI score) reported by the patients showed a very low negative correlation (correlation coefficient = −0.14299), indicating that there was not much correlation. That is, the cutaneous response was not sufficient to explain the benefit obtained by the patient from treatment (Fig. 5). In contrast, the dermatologists’ evaluation of general success of apremilast and the patient perception of treatment benefit (overall PBI score) showed a high positive correlation (correlation coefficient = 0.63629). The cases in which the dermatologists indicated that their expectations were achieved or exceeded coincided with the patient perception of high benefit (PBI ≥ 3), and vice versa.
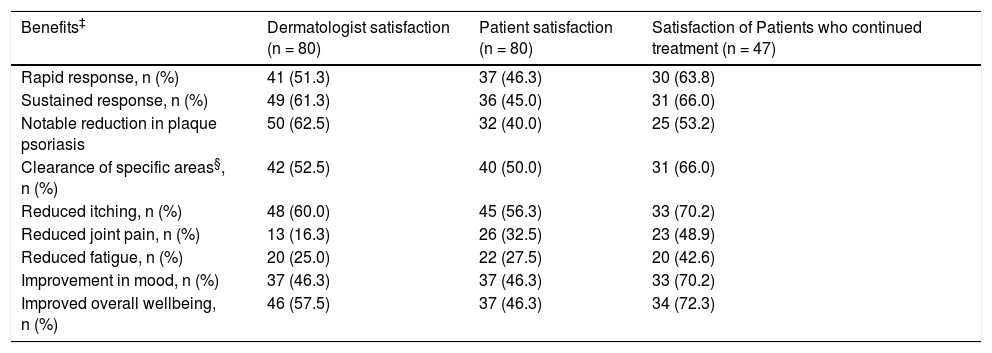
Table 4 shows the levels of dermatologist and patient satisfaction with respect to effect of apremilast treatment on symptoms. Specifically, the satisfaction of patients who continued in treatment with apremilast was 63.8% in rapid response, 66.0% in sustained response, 70.2% in reduced itching, 70.2% in improvement in mood, and 72.3% in overall wellbeing.
Levels of dermatologist and patient satisfaction with respect to effect of apremilast treatment on symptoms (data from specific questionnaires).
| Benefits‡ | Dermatologist satisfaction (n = 80) | Patient satisfaction (n = 80) | Satisfaction of Patients who continued treatment (n = 47) |
|---|---|---|---|
| Rapid response, n (%) | 41 (51.3) | 37 (46.3) | 30 (63.8) |
| Sustained response, n (%) | 49 (61.3) | 36 (45.0) | 31 (66.0) |
| Notable reduction in plaque psoriasis | 50 (62.5) | 32 (40.0) | 25 (53.2) |
| Clearance of specific areas§, n (%) | 42 (52.5) | 40 (50.0) | 31 (66.0) |
| Reduced itching, n (%) | 48 (60.0) | 45 (56.3) | 33 (70.2) |
| Reduced joint pain, n (%) | 13 (16.3) | 26 (32.5) | 23 (48.9) |
| Reduced fatigue, n (%) | 20 (25.0) | 22 (27.5) | 20 (42.6) |
| Improvement in mood, n (%) | 37 (46.3) | 37 (46.3) | 33 (70.2) |
| Improved overall wellbeing, n (%) | 46 (57.5) | 37 (46.3) | 34 (72.3) |
‡The count and percentage for the response “agree” or “fully agree” are shown for the question to the dermatologist “How do you think that apremilast has helped in psoriasis treatment in this patient?” and to the question to the patient “how do you think that apremilast has helped in the treatment of psoriasis?”.
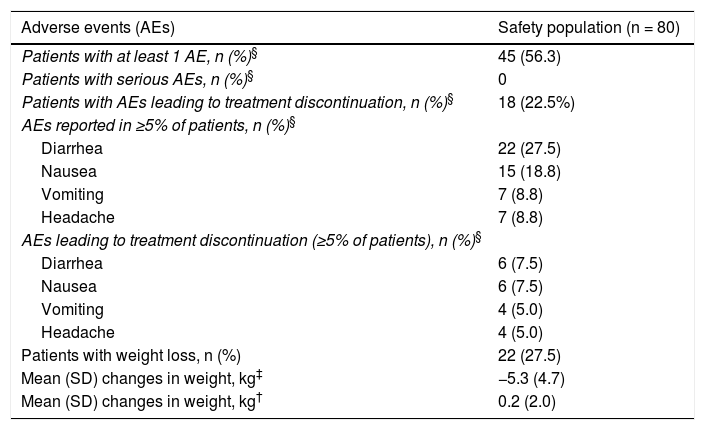
A total of 80 AEs were reported in 45 patients (56.3%). In most cases (n = 44 [55.0%]), these AEs were treatment related, and in 18 patients (22.5%) they led to withdrawal of treatment. The most frequently reported AEs were diarrhea (27.5%) and nauseas (18.8%) (Table 5). Three patients suffered insomnia (one of these had received prior treatment for anxiety). No serious AEs were reported.
Safety with apremilast.
| Adverse events (AEs) | Safety population (n = 80) |
|---|---|
| Patients with at least 1 AE, n (%)§ | 45 (56.3) |
| Patients with serious AEs, n (%)§ | 0 |
| Patients with AEs leading to treatment discontinuation, n (%)§ | 18 (22.5%) |
| AEs reported in ≥5% of patients, n (%)§ | |
| Diarrhea | 22 (27.5) |
| Nausea | 15 (18.8) |
| Vomiting | 7 (8.8) |
| Headache | 7 (8.8) |
| AEs leading to treatment discontinuation (≥5% of patients), n (%)§ | |
| Diarrhea | 6 (7.5) |
| Nausea | 6 (7.5) |
| Vomiting | 4 (5.0) |
| Headache | 4 (5.0) |
| Patients with weight loss, n (%) | 22 (27.5) |
| Mean (SD) changes in weight, kg‡ | −5.3 (4.7) |
| Mean (SD) changes in weight, kg† | 0.2 (2.0) |
Abbreviation: AE, adverse event.
The observational APPRECIATE study shows that patients treated with apremilast in a clinical practice setting in Spain had different characteristics to those reported in clinical trials2,3,5: more moderate skin involvement (mean PASI score = 8.3 with scores <10 in 78.6% of patients) along with a substantial impact on QoL (mean DLQI score = 8.9). Prior publications (including that of the European study population for APPRECIATE) had already confirmed this tendency to treat patients with less severe disease, even though the impact on QoL is high2,3,5,6,11–16. This observation suggests that apremilast can satisfy the need for treatment in patients with a more moderate skin involvement.
Although the PASI and DLQI are commonly used tools for assessing disease severity and treatment efficacy, in the present study, they could only be collected at the start of treatment and at follow-up in 37.5% and 15.0% of patients, respectively. These data suggest that these scales are not consistently used in routine clinical practice, and so it appears necessary to improve the assessment of the patient needs and treatment effectiveness beyond the traditional outcome measures. The present analysis shows a low negative correlation between percentage change in PASI at 6 months of treatment and overall patient-reported treatment benefit (PBI), suggesting that improvement in visible lesions was not sufficient to explain patient benefit. Furthermore, patients also reported their satisfaction with rapid and sustained response to apremilast, as well as with reduced itching, mood improvement, and overall wellbeing. Dermatologists and patients reported a similar satisfaction with apremilast. Furthermore, the dermatologist assessment of the general success of apremilast in meeting expectations and patient perception of benefit obtained showed a high positive correlation.
The group of patients who remained on treatment with apremilast for 6 months showed an improvement in PASI (reduction of 5.5 points), BSA (reduction of 10.6%), DLQI (reduction of 6.2 points), PBI (all patients achieved PBI ≥ 1, the value considered as significant clinical benefit)9, and TSQM-9 (overall satisfaction = 70.5 points). The values achieved for PASI (3.4), BSA (2.6), and DLQI (2.2) were consistent with those observed in the Spanish Psoriasis Study Group (abbreviated as GPs in Spanish) (3.6; 3.1 [week 12]/2.5 [week 52]; 5.2 [week 12]/2.3 [week 52], respectively)16 and the interim analysis of the APPROPRIATE study (2.6; 3.7 and 53% with values 0–1, respectively)17. In the multinational APPRECIATE study, the values obtained (PASI = 4.6; BSA = 10.9; DLQI = 5.7) were slightly higher, but it should also be remembered that the starting values were also higher (PASI = 12.5; BSA = 25.4; DLQI = 13.4)6. Persistence at 6 months was 58.8% in this study compared with 72.3% observed in the multinational APPRECIATE study.
The safety profile of apremilast confirmed that already observed in the clinical trials2,3,5. As with other studies in clinical practice, the proportion of AEs was lower than in clinical trials (47%–59% versus 67%–79%, respectively) but, in contrast, the proportion of patients who discontinued apremilast due to AEs was higher (16%–23% versus 6.6%–7.3%)2,3,5,16,18,19. Although the Summary of Product Characteristics of apremilast urges caution in patients with depression1, 11.3% of patients treated in the present study were receiving antidepressants when they started apremilast; none of these patients had AEs classed as psychiatric disorders. On the other hand, in the present study, upper airway infections did not occur although such events were reported in 8.4%, mainly of mild severity, in the Summary of Product Characteristics1.
In this study, all consecutive patients who could be contacted 6 (±1) months after starting treatment were included (regardless of whether patients had completed the 6 months of treatment with apremilast) with the aim of minimizing patient selection bias. However, the study does present certain limitations inherent to its retrospective design, its short follow-up period, and clinical practice conditions, as well as the lack of a control group and missing patient data in some cases. The low percentage of patients with PASI and DLQI values available could have had an impact on the analyses performed. A potential positive selection bias is acknowledged because patients with a positive experience with apremilast may be more willing to agree to participate in the study. Finally, as the questionnaires were retrospectively filled out, we cannot rule out the possibility of recall bias, although the study duration (6 months) was chosen with a view to reducing the risk of such a bias.
ConclusionsThe APPRECIATE study shows that patients who started apremilast treatment in clinical practice in Spain had a lower skin involvement than those recruited in the clinical trials. Even so, an unexpectedly high impact on the QoL was found in the study participants. This suggests that, when assessing the true importance of disease, other factors beside the extent of skin involvement should be taken into account.
The study findings showed that after 6 months of apremilast treatment, improvements were observed in the overall assessments of disease severity (PASI75 36.7%), QoL (mean DLQI decreased 6 points), satisfaction of patients who continued in treatment with apremilast (63.8% in rapid response, 66.0% in sustained response, 70.2% in reduction of pruritus, 70.2% in improvement in mood, and 72.3% in overall wellbeing).
Finally, the results of this study show the need for a more comprehensive definition of the severity of psoriasis, incorporating patient-centered measures to the traditional scales used.
FundingThis article was funded by Celgene. Amgen acquired the global rights to Otezla® (apremilast) on November 21, 2019.
Qualified researchers can request data from clinical trials sponsored by Amgen. Full details are available from http://www.amgen.com/datasharing.
Conflicts ofinterestPedro Herranz: investigator/consultant/speaker for AbbVie, Almirall, Amgen, Janssen, LEO Pharma, Lilly, Novartis, Pfizer, Sandoz, Sanofi, and UCB Pharma.
Lidia Trasobares: investigator and/or speaker for AbbVie, Almirall, Amgen, Celgene, Janssen, LEO Pharma, Meda, and Novartis.
Almudena Mateu: no conflicts of interest declared.
Esperanza Martínez: no conflicts of interest declared.
Ricardo Ruiz Villaverde: speaker and/or consultant for AbbVie, Amgen, Celgene, LEO Pharma, Lilly, MSD, Novartis, and UCB Pharma.
Ofelia Baniandrés: consultant and/or speaker for AbbVie, Celgene, LEO Pharma, Lilly MSD, Novartis, Janssen, and Pfizer.
Javier Mataix Díaz: no conflicts of interest declared.
Natalia Jiménez Gómez: no conflicts of interest declared.
Marta Serra: speaker for AbbVie, Almirall, Celgene, Janssen, LEO Pharma, and Lilly.
Diana Patricia Ruiz Genao: consultant and/or honorary speaker for AbbVie, Amgen, Janssen, LEO Pharma, Lilly, Novartis, and Pfizer.
Noelia Rivera: no conflicts of interest declared.
Jesús Tercedor Sánchez: no conflicts of interest declared.
Carmen García: former employee of Amgen.
Myriam Cordey: employee of Amgen (Europe) GmbH.
Enrique Herrera Acosta: no conflicts of interest declared.
The authors would like to thank the investigators, coordinators, and personnel of the participating centers, as well as the patients for participating in this study. Medical writing support was provided by Irene Mansilla, MSc, of TFS S.L.
Please cite this article as: Herranz P, Trasobares L, Mateu A, Martínez E, Ruiz-Villaverde R, Baniandrés O, et al. Caracterización y resultados de pacientes tratados con apremilast en la práctica clínica habitual española: resultados del estudio APPRECIATE. Actas Dermosifiliogr. 2021;112:817–827.