There are several therapeutic options for infantile haemangiomas (IH). Propranolol is used according to a pivotal trial. We aimed to describe the characteristics of IH in clinical practice, including the therapies used, and to compare the characteristics of patients treated with propranolol with those of the trial to assess its external validity.
MethodsConsecutive patients attending 12 Spanish hospitals from June 2016 to October 2019 were included (n = 601).
ResultsThe mean age was 3.9 (SD: 1.9) months, with a 2:1 female-to-male ratio. Most IHs were localized (82%, 495), superficial (64%, 383) and located in the face (25%, 157) and trunk (31%, 188). Median size was 17 (IR: 10–30) × 12 (IR: 7–20) mm. Complications were found in 16 (3%) patients. Treatment was initiated for 52% (311). Most patients received timolol (76%, 237); propranolol was reserved for complications or high-risk IHs. Aesthetic impairment was the main reason for starting therapy (64%, 199). Several characteristics of the patients and IHs treated with propranolol are similar to those of the pivotal clinical trial, but 1/3 of IHs did not reach the minimum diameter to meet the inclusion criteria, and important prognostic information was not reported.
ConclusionsAs most patients receive treatment for aesthetic impairment, there is a need to better understand the aesthetic results of therapies and to increase evidence on the use of timolol, which is currently the most common therapy. Propranolol is being used in a population generally similar to that of the trial; however, this statement cannot be definitely confirmed.
Existen diversas opciones terapéuticas para los hemangiomas infantiles (HI). El propranolol se utiliza con base en un ensayo pivotal. Nuestro objetivo fue describir las características del HI en la práctica clínica, incluyendo las terapias utilizadas, así como comparar las características de los pacientes tratados con propranolol y las de los pacientes del ensayo, para valorar su validez externa.
MétodosSe incluyó consecutivamente a los pacientes que acudieron a doce hospitales españoles desde junio de 2016 a octubre de 2019 (n = 601).
ResultadosLa edad media fue de 3,9 (DE: 1,9) meses, con una ratio mujer-varón de 2:1. La mayoría de los HI fueron de tipo localizado (82%, 495), superficial (64%, 383) y ubicados en cara (25%, 157) y tronco (31%, 188). El tamaño mediano fue de 17 (RI: 10–30) × 12 (RI: 7–20) mm. Se encontraron complicaciones en 16 (3%) pacientes. Se inició tratamiento en el 52% (311) de los casos. La mayoría de los pacientes recibió timolol (76%, 237), reservándose propranolol para las complicaciones o los HI de alto riesgo. El compromiso estético fue el principal motivo de iniciar la terapia (64%, 199). Las diversas características de los pacientes y de los HI tratados con propranolol fueron similares a las del ensayo clínico pivotal, aunque 1/3 de los HI no alcanzó el diámetro mínimo para cumplir los criterios de inclusión, y no se comunicó información pronóstica importante.
ConclusionesDado que muchos pacientes reciben tratamiento debido al compromiso estético, existe una necesidad de conocer mejor los resultados estéticos de las terapias e incrementar la evidencia sobre el uso de timolol, que actualmente es la terapia más común. El propranolol está siendo utilizado en una población generalmente similar a la del ensayo; sin embargo, esta afirmación no puede confirmarse de manera definitiva.
Infantile haemangiomas (IHs) are the most common soft-tissue tumours of infancy1, with a female-to-male ratio ranging from 2:1 to 5:12,3. The prevalence is estimated to be approximately 10% of newborns2. One of the main locations of IHs is the head and neck2,4–8, with subsequent aesthetic implications. In certain locations, IHs may even interfere with the development of vital functions or affect the quality of life of both the children and their parents9–11. Approximately 10% of IHs develop ulcerations, especially those with superficial components12,13.
Although all IHs involute over time, between 46–69% can leave sequelae in the absence of treatment14–16, such as fibrofatty tissue, anetoderma, atrophy, telangiectasias, changes in skin coloration or scars if ulceration has occurred. Most studies evaluating sequelae or epidemiological characteristics of IHs were carried out prior to the use of propranolol, which has changed the treatment paradigm for these lesions once its effectiveness was shown in a clinical trial and its use was approved for the treatment of IHs17. However, although aesthetic impairment seems to be an important reason to start treatment with propranolol, in the trial, complete or nearly complete responses to treatment were assessed, but the possible sequelae after treatment were not analysed, and aesthetic impairment was not an inclusion criterion. Moreover, previous studies found that 91% of patients treated with propranolol presented with sequelae, and 74% presented with severe sequelae, although the inclusion criteria were restrictive and propranolol dosages suboptimal18.
We decided to start a Spanish prospective cohort of IHs given the changing clinical context of this common tumour that may seriously affect children, the lack of good descriptions of the involuting phase and possible sequelae in long-term prospective studies19,20, and the appearance of a new effective treatment.
The aims of the present article were 1) to describe IHs as seen in clinical practice, including demographic and clinical characteristics and therapies used, and 2) to compare our data on patients treated with propranolol in common practice with those of the pivotal randomized clinical trial17 to assess the external validity of the trial.
Patients and methodsDesignThe Spanish Academy of Dermatology and Venereology (AEDV) began a nationwide prospective cohort of IHs in June 2016, and the study period extended to October 2019. Patients were recruited from 12 Spanish hospitals with paediatric dermatology departments. A 5-year follow-up study was initially planned and is still ongoing. This paper describes the baseline data of the cohort.
Inclusion criteriaNew-borns younger than 9 months with one or more IHs diagnosed by their characteristic clinical features or, in case of doubt, by immunohistochemical GLUT-1 staining were recruited. All consecutive patients attending one of the participating hospitals were included regardless of the proposed therapy (clinical observation or treatment). As this is a clinic-based observational study, the therapeutic approach was decided during routine clinical practice.
Exclusion criteriaThe following patients were excluded: patients whose family was planning a short-term move that would make follow-up difficult; patients who had already started any kind of treatment for IHs at the moment of the baseline visit; and patients who refused to participate in the study.
Variables of interestMaternal obstetric history, demographic and biometric characteristics of the patients, IH locations and characteristics, such as morphologic and clinical subtypes, size, height or the kind of surface and edge, the presence of complications or likelihood of complications and treatment initiation and the reasons for starting therapy were recorded at baseline and constitute the data used for this paper. IHs were classified according to their distribution (morphologic subtypes: localized, indeterminate, segmental and multifocal) and to their deepness and edge (clinical subtypes: superficial, mixed-sessile, mixed-pedunculated, deep and abortive). Complications were defined as the presence of any of the following: ocular, airway or visceral involvement, ulcerations or functional impairment (visual, nasal, feeding, airway, torticollis, external auditory canal obstruction or any other function considered important by the physician). Definitions were recorded in the study protocol, and a photographic atlas was created to ensure homogeneity in the collection of data on IH characteristics.
Data were collected by previously trained physicians (the dermatologists responsible for the paediatric dermatology departments at each participating hospital) using an online data collection system (Openclinica 3.1). Quality control consisted of continuous monthly online monitoring and annual on-site monitoring from a simple random sample of 10 patients from each of the hospitals (approx. 20% of the included patients per year).
Statistical analysisDescriptive statistics were used to explore the characteristics of the sample. The Shapiro–Wilk test and histograms were used to explore the normality of the variables. Continuous variables are expressed as the mean and standard deviation (SD) or median (25th–75th percentiles). For qualitative variables, absolute and relative frequencies were estimated. Differences between qualitative variables were assessed by means of the χ2 test or Fisher’s exact test, when necessary. Differences between continuous variables were assessed by means of Student’s t-test or the Mann–Whitney U test. Statistical analyses were performed using STATA statistical software (version 16.0; Statacorp, College Station, TX, USA).
EthicsThe study protocol was approved by the institutional review board of Hospital Santa Creu i Sant Pau (Barcelona, 16/079). All patients’ parents or caregivers signed written informed consent at the time of enrolment.
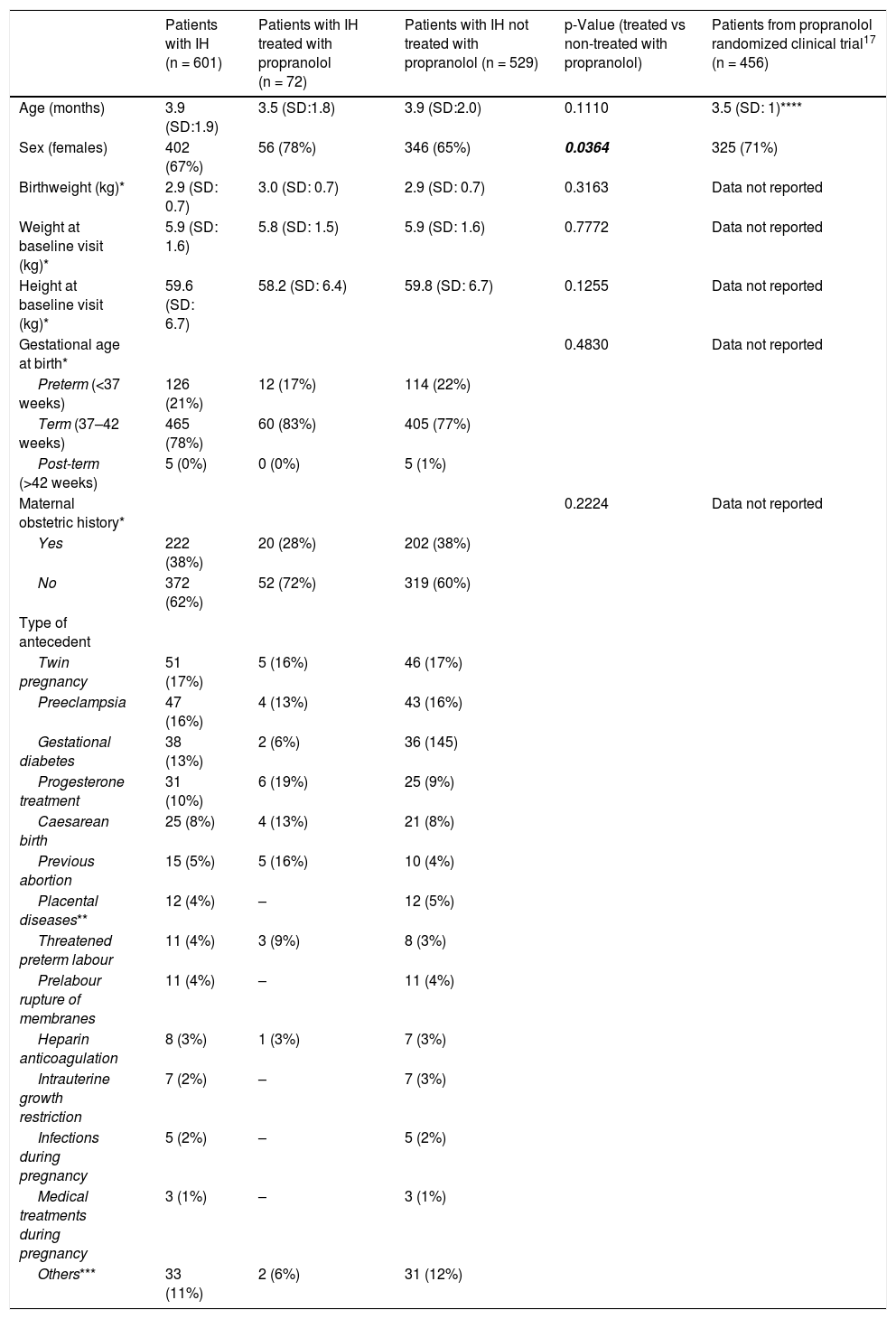
ResultsBaseline characteristicsThe baseline characteristics of the sample are shown in Table 1. The mean age was approximately 4 months; distinguishing between clinical IH subtypes, the mean age of deep IH patients was 4.5 months, while in patients with IH with superficial components, it was 3.8 months (p = 0.0075). There was a higher proportion of females (ratio 2:1). The mean weight and height at the baseline visit were normal for age, as was the weight at birth. The majority of new-borns were full-term. More than 60% of mothers did not have any abnormal maternal obstetric history. Among those who had obstetric antecedents, twin pregnancy, preeclampsia, gestational diabetes, and treatment with progesterone were the most prevalent. Patients whose mothers were treated with progesterone showed no differences in complications, median size, or proportion of segmental IH from those whose mothers did not receive progesterone.
Baseline characteristics of the sample.
| Patients with IH (n = 601) | Patients with IH treated with propranolol (n = 72) | Patients with IH not treated with propranolol (n = 529) | p-Value (treated vs non-treated with propranolol) | Patients from propranolol randomized clinical trial17 (n = 456) | |
|---|---|---|---|---|---|
| Age (months) | 3.9 (SD:1.9) | 3.5 (SD:1.8) | 3.9 (SD:2.0) | 0.1110 | 3.5 (SD: 1)**** |
| Sex (females) | 402 (67%) | 56 (78%) | 346 (65%) | 0.0364 | 325 (71%) |
| Birthweight (kg)* | 2.9 (SD: 0.7) | 3.0 (SD: 0.7) | 2.9 (SD: 0.7) | 0.3163 | Data not reported |
| Weight at baseline visit (kg)* | 5.9 (SD: 1.6) | 5.8 (SD: 1.5) | 5.9 (SD: 1.6) | 0.7772 | Data not reported |
| Height at baseline visit (kg)* | 59.6 (SD: 6.7) | 58.2 (SD: 6.4) | 59.8 (SD: 6.7) | 0.1255 | Data not reported |
| Gestational age at birth* | 0.4830 | Data not reported | |||
| Preterm (<37 weeks) | 126 (21%) | 12 (17%) | 114 (22%) | ||
| Term (37–42 weeks) | 465 (78%) | 60 (83%) | 405 (77%) | ||
| Post-term (>42 weeks) | 5 (0%) | 0 (0%) | 5 (1%) | ||
| Maternal obstetric history* | 0.2224 | Data not reported | |||
| Yes | 222 (38%) | 20 (28%) | 202 (38%) | ||
| No | 372 (62%) | 52 (72%) | 319 (60%) | ||
| Type of antecedent | |||||
| Twin pregnancy | 51 (17%) | 5 (16%) | 46 (17%) | ||
| Preeclampsia | 47 (16%) | 4 (13%) | 43 (16%) | ||
| Gestational diabetes | 38 (13%) | 2 (6%) | 36 (145) | ||
| Progesterone treatment | 31 (10%) | 6 (19%) | 25 (9%) | ||
| Caesarean birth | 25 (8%) | 4 (13%) | 21 (8%) | ||
| Previous abortion | 15 (5%) | 5 (16%) | 10 (4%) | ||
| Placental diseases** | 12 (4%) | – | 12 (5%) | ||
| Threatened preterm labour | 11 (4%) | 3 (9%) | 8 (3%) | ||
| Prelabour rupture of membranes | 11 (4%) | – | 11 (4%) | ||
| Heparin anticoagulation | 8 (3%) | 1 (3%) | 7 (3%) | ||
| Intrauterine growth restriction | 7 (2%) | – | 7 (3%) | ||
| Infections during pregnancy | 5 (2%) | – | 5 (2%) | ||
| Medical treatments during pregnancy | 3 (1%) | – | 3 (1%) | ||
| Others*** | 33 (11%) | 2 (6%) | 31 (12%) |
Continuous variables are expressed as the mean (standard deviation). Qualitative variables are expressed as absolute (relative) frequencies. Statistically significant p values are in bold.
This category includes threatened abortion (6), hypothyroidism (6), high blood pressure (4), induced labour (3), intrahepatic cholestasis of pregnancy (3), hyperthyroidism (2), lupus (2), HELLP syndrome (2), Crohn’s disease (1), drug abuse (1), renal disease (1), multiple sclerosis (1), pneumothorax (1) and amniotic sac prolapse (1). Note that there were mothers with several antecedent complications.
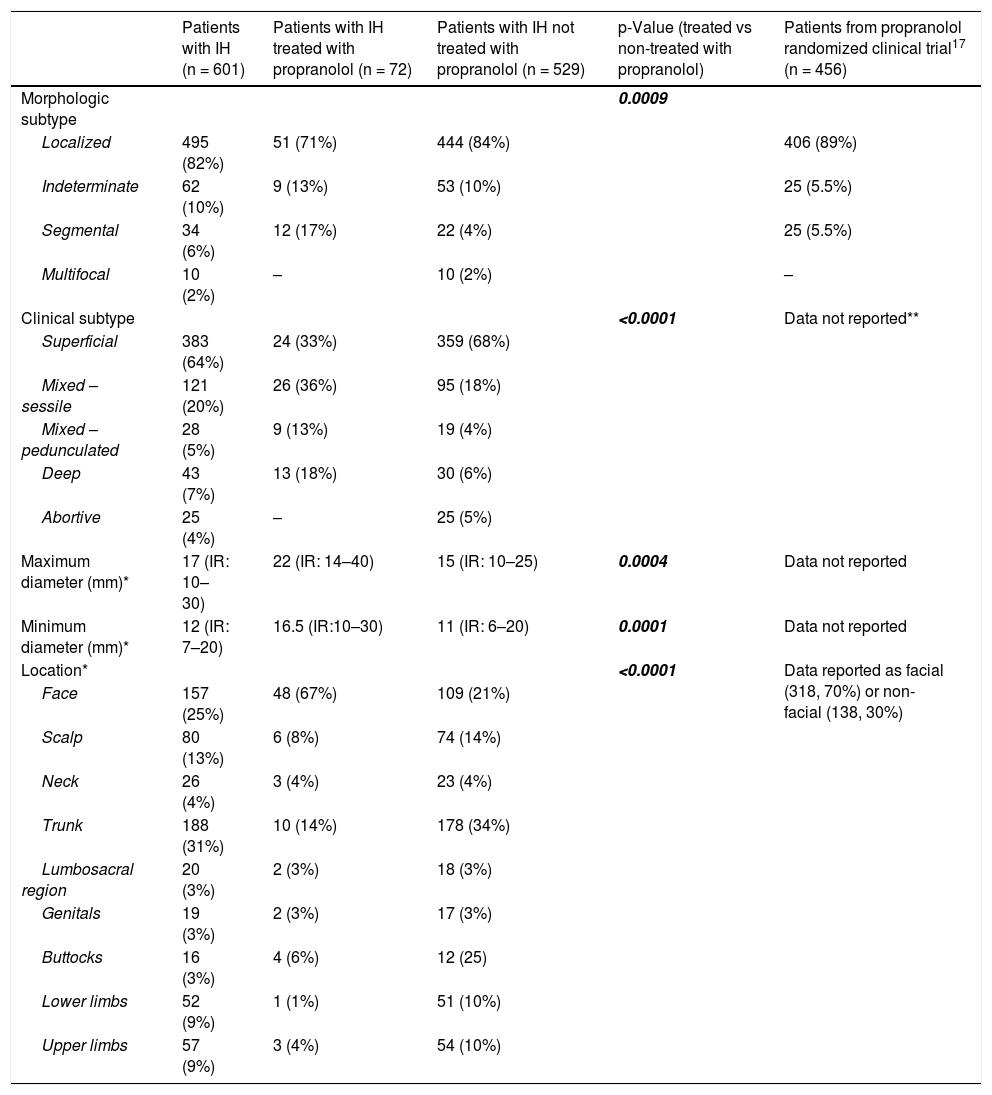
The characteristics and locations of the IHs are shown in Table 2. Most IHs were localized and superficial. The median size (maximum × minimum diameter) was 17 mm × 12 mm. The most frequent locations were the trunk and face. Among haemangiomas with superficial components, superficial IHs more frequently had a steep edge, while mixed IHs were more likely to have a progressive edge (p < 0.05). Most of the IHs had a smooth surface. The height of the superficial component was greater in mixed IHs (p < 0.0001).
Infantile haemangiomas characteristics and locations.
| Patients with IH (n = 601) | Patients with IH treated with propranolol (n = 72) | Patients with IH not treated with propranolol (n = 529) | p-Value (treated vs non-treated with propranolol) | Patients from propranolol randomized clinical trial17 (n = 456) | |
|---|---|---|---|---|---|
| Morphologic subtype | 0.0009 | ||||
| Localized | 495 (82%) | 51 (71%) | 444 (84%) | 406 (89%) | |
| Indeterminate | 62 (10%) | 9 (13%) | 53 (10%) | 25 (5.5%) | |
| Segmental | 34 (6%) | 12 (17%) | 22 (4%) | 25 (5.5%) | |
| Multifocal | 10 (2%) | – | 10 (2%) | – | |
| Clinical subtype | <0.0001 | Data not reported** | |||
| Superficial | 383 (64%) | 24 (33%) | 359 (68%) | ||
| Mixed – sessile | 121 (20%) | 26 (36%) | 95 (18%) | ||
| Mixed – pedunculated | 28 (5%) | 9 (13%) | 19 (4%) | ||
| Deep | 43 (7%) | 13 (18%) | 30 (6%) | ||
| Abortive | 25 (4%) | – | 25 (5%) | ||
| Maximum diameter (mm)* | 17 (IR: 10–30) | 22 (IR: 14–40) | 15 (IR: 10–25) | 0.0004 | Data not reported |
| Minimum diameter (mm)* | 12 (IR: 7–20) | 16.5 (IR:10–30) | 11 (IR: 6–20) | 0.0001 | Data not reported |
| Location* | <0.0001 | Data reported as facial (318, 70%) or non-facial (138, 30%) | |||
| Face | 157 (25%) | 48 (67%) | 109 (21%) | ||
| Scalp | 80 (13%) | 6 (8%) | 74 (14%) | ||
| Neck | 26 (4%) | 3 (4%) | 23 (4%) | ||
| Trunk | 188 (31%) | 10 (14%) | 178 (34%) | ||
| Lumbosacral region | 20 (3%) | 2 (3%) | 18 (3%) | ||
| Genitals | 19 (3%) | 2 (3%) | 17 (3%) | ||
| Buttocks | 16 (3%) | 4 (6%) | 12 (25) | ||
| Lower limbs | 52 (9%) | 1 (1%) | 51 (10%) | ||
| Upper limbs | 57 (9%) | 3 (4%) | 54 (10%) |
| Superficial subtype | Mixed subtype | Superficial subtype | Mixed subtype | Superficial subtype | Mixed subtype | |||
|---|---|---|---|---|---|---|---|---|
| Edge of the superficial component* | 0.0395 | Data not reported | ||||||
| Steep | 171 (46%) | 52 (37%) | 9 (38%) | 9 (26%) | 162 (47%) | 43 (40%) | ||
| Progressive | 199 (54%) | 90 (63%) | 15 (62%) | 25 (74%) | 184 (53%) | 65 (60%) | ||
| Surface of the superficial component* | 0.0020 | Data not reported | ||||||
| Smooth | 249 (68%) | 102 (73%) | 20 (87%) | 26 (87%) | 229 (67%) | 76 (69%) | ||
| Cobblestone | 116 (32%) | 38 (27%) | 3 (13%) | 4 (13%) | 113 (33%) | 34 (31%) | ||
| Height of the superficial component* | 0.0172 | Data reported as slight (114, 25%), moderate (148, 33%) or marked (157, 34%) | ||||||
| <1 mm | 166 (46%) | 53 (38%) | 7 (29%) | 16 (50%) | 159 (48%) | 37 (34%) | ||
| 1-2 mm | 133 (37%) | 43 (31%) | 8 (33%) | 5 (15%) | 125 (38%) | 38 (35%) | ||
| 2-5 mm | 57 (16%) | 29 (20%) | 7 (29%) | 8 (25%) | 50 (15%) | 21 (19%) | ||
| >5 mm | 2 (1%) | 15 (11%) | 2 (8%) | 3 (9%) | – | 12 (11%) |
Continuous variables are expressed as medians (25th–75th percentiles). Qualitative variables are expressed as absolute (relative) frequencies. Statistically significant p values are in bold.
Colour was evaluated as bright red in 460 (78%) IHs, pale pink in 84 (14%), barely perceptible in 32 (6%) and imperceptible in 11 (2%).
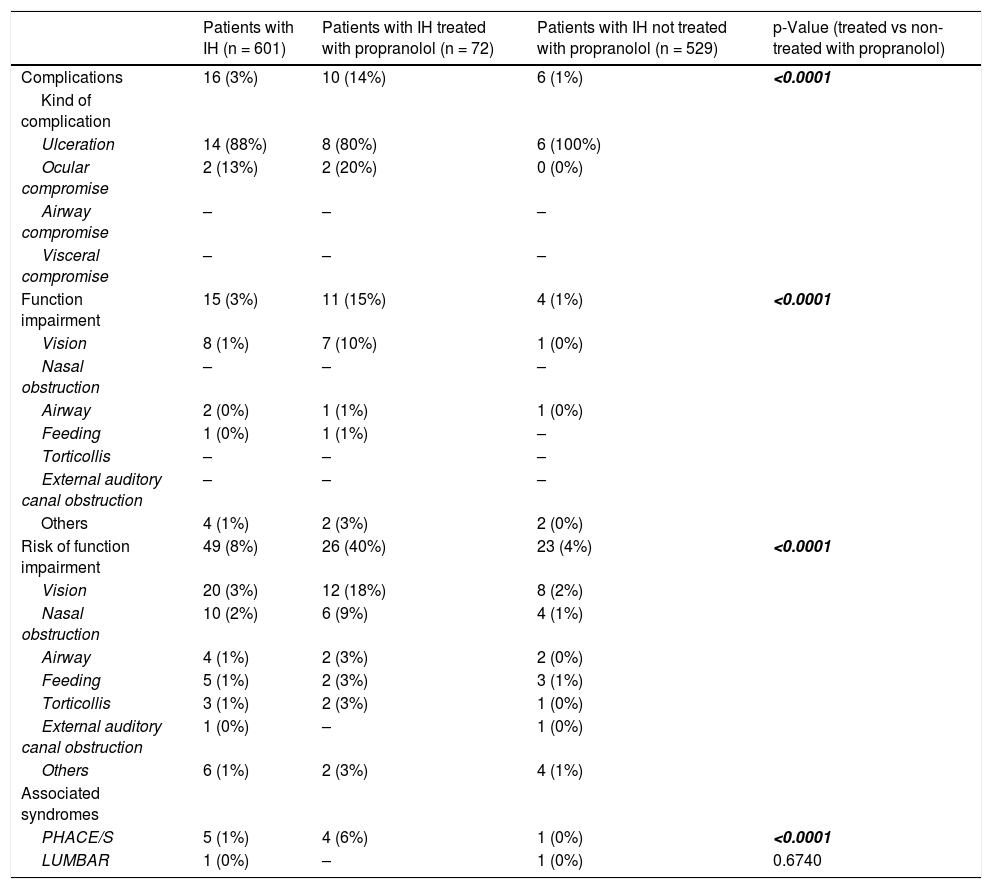
ComplicationsOnly 16 (3%) patients presented with complications at the baseline visit, mostly ulcerations, and 15 (3%) presented with functional impairment, mainly visual impairment (Table 3). Forty-nine (8%) patients presented with a risk of functional impairment, with vision being again the most compromised function. Five (1%) patients presented with PHACE syndrome, and 1 (0%) patient presented with LUMBAR syndrome.
Complications, functional impairment, risk of functional impairment and associated syndromes of IH.
| Patients with IH (n = 601) | Patients with IH treated with propranolol (n = 72) | Patients with IH not treated with propranolol (n = 529) | p-Value (treated vs non-treated with propranolol) | |
|---|---|---|---|---|
| Complications | 16 (3%) | 10 (14%) | 6 (1%) | <0.0001 |
| Kind of complication | ||||
| Ulceration | 14 (88%) | 8 (80%) | 6 (100%) | |
| Ocular compromise | 2 (13%) | 2 (20%) | 0 (0%) | |
| Airway compromise | – | – | – | |
| Visceral compromise | – | – | – | |
| Function impairment | 15 (3%) | 11 (15%) | 4 (1%) | <0.0001 |
| Vision | 8 (1%) | 7 (10%) | 1 (0%) | |
| Nasal obstruction | – | – | – | |
| Airway | 2 (0%) | 1 (1%) | 1 (0%) | |
| Feeding | 1 (0%) | 1 (1%) | – | |
| Torticollis | – | – | – | |
| External auditory canal obstruction | – | – | – | |
| Others | 4 (1%) | 2 (3%) | 2 (0%) | |
| Risk of function impairment | 49 (8%) | 26 (40%) | 23 (4%) | <0.0001 |
| Vision | 20 (3%) | 12 (18%) | 8 (2%) | |
| Nasal obstruction | 10 (2%) | 6 (9%) | 4 (1%) | |
| Airway | 4 (1%) | 2 (3%) | 2 (0%) | |
| Feeding | 5 (1%) | 2 (3%) | 3 (1%) | |
| Torticollis | 3 (1%) | 2 (3%) | 1 (0%) | |
| External auditory canal obstruction | 1 (0%) | – | 1 (0%) | |
| Others | 6 (1%) | 2 (3%) | 4 (1%) | |
| Associated syndromes | ||||
| PHACE/S | 5 (1%) | 4 (6%) | 1 (0%) | <0.0001 |
| LUMBAR | 1 (0%) | – | 1 (0%) | 0.6740 |
Data are expressed as absolute (relative) frequencies. Statistically significant p values are in bold.
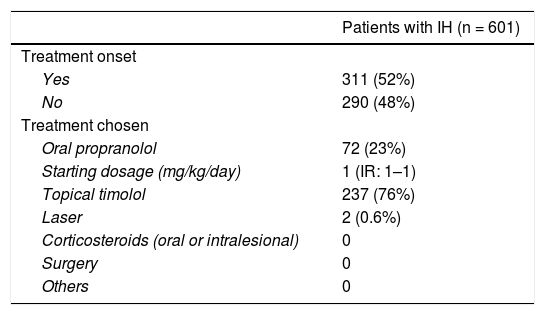
The onset of and reasons for treatment are shown in Table 4. Slightly more than half of the patients started treatment at the baseline visit. Of these patients, approximately 3/4 started with topical timolol (5 mg/ml applied twice a day; data were available for 80%, but since this is the posology recommended in previous research21, it was expected that this posology would be used for most patients), and approximately 1/4 started with oral propranolol (median initial dosage: 1 mg/kg/day, as recommended by the European Medicine Agency22; data on starting dose was available for 76%, but it is expected that most patients started this dosage). Only 2 patients started laser therapy, and there were no other treatments.
Treatment onset and its causes.
| Patients with IH (n = 601) | |
|---|---|
| Treatment onset | |
| Yes | 311 (52%) |
| No | 290 (48%) |
| Treatment chosen | |
| Oral propranolol | 72 (23%) |
| Starting dosage (mg/kg/day) | 1 (IR: 1–1) |
| Topical timolol | 237 (76%) |
| Laser | 2 (0.6%) |
| Corticosteroids (oral or intralesional) | 0 |
| Surgery | 0 |
| Others | 0 |
| Patients with IH who received treatment (n = 311) | Patients with IH treated with propranolol (n = 72) | Patients with IH treated with timolol (n = 237) | |
|---|---|---|---|
| Causes of treatment onset | |||
| Vision impairment | 21 (6.8%) | 19 (26%) | 2 (0.8%) |
| Haemangioma in visual field | 7 (2.3%) | 6 (8.3%) | 1 (0.4%) |
| Palpebral closure without pupil occlusion | 12 (3.9%) | 11 (15%) | 1 (0.4%) |
| Palpebral closure with pupil occlusion | 1 (0.3%) | 1 (1.4%) | – |
| Eyeball compression without deviation | 1 (0.3%) | 1 (1.4%) | – |
| Airway impairment | 4 (1.2%) | 4 (5.6%) | – |
| Asymptomatic airway IH | 2 (0.6%) | 2 (2.8%) | – |
| IH causing stridor | 2 (0.6%) | 2 (2.8%) | – |
| Aesthetic impairment | 199 (64%) | 57 (79%) | 140 (52%) |
| Low impairment | 141 (45%) | 19 (26%) | 121 (51%) |
| Moderate impairment | 55 (18%) | 35 (49%) | 19 (8%) |
| Severe impairment | 3 (1%) | 3 (4.2%) | – |
| Ulceration | 13 (4.2%) | 10 (14%) | 1 (0.4%) |
| <10% | 3 (1%) | 2 (2.8%) | 1 (0.4%) |
| 10-25% | 5 (1.6%) | 4 (5.6%) | – |
| 25-50% | 4 (1.3%) | 4 (5.6%) | – |
| >50% | 1 (0.3%) | – | |
| Pain | 8 (2.6%) | 6 (8.3%) | – |
| Mild discomfort | 3 (1%) | 2 (2.8%) | – |
| Pain awakens the patient | 4 (1.3%) | 3 (4.2%) | – |
| Analgesia required | 1 (0.3%) | 1 (1.4%) | – |
| None of the above or not reported | 103 (33%) | 7 (9.7%) | 96 (41%) |
Continuous variables are expressed as medians (25th–75th percentiles). Qualitative variables are expressed as absolute (relative) frequencies.
In terms of sociodemographic characteristics, a higher proportion of females started treatment with propranolol (Table 1). Compared with that of the pivotal trial, age was similar, and the female-to-male ratio was higher; the other data were not reported in the trial.
Regarding IH characteristics, patients with segmental IHs, mixed and deep IHs, IHs with a larger diameter, a higher height, a progressive border, a smooth surface, and IHs located on the face were more prone to start treatment with propranolol. Compared with the trial population, higher proportions of indeterminate and segmental IHs treated with propranolol and similar proportions of superficial IHs and facial IHs. Other data about IH characteristics were not reported in the trial (Table 2).
Patients who suffered complications, functional impairment, or risk of functional impairment and those with PHACE syndrome started treatment with propranolol more frequently (Table 3). In the trial, data about complications were not reported.
The main reason for starting treatment, both with propranolol (79%) and with timolol (52%), was aesthetic impairment (Table 4). Only 47 (66%) patients treated with propranolol and 260 (50%) patients not treated with propranolol had an IH diameter larger than 15 mm (which was an inclusion criterion for the trial). Patients who started treatment because of aesthetic impairment were more likely to have received timolol (140, 70%) than propranolol (57, 29%). Information about the causes of treatment onset was not reported in the clinical trial.
CommentIn this study, we report the baseline characteristics of the AEDV cohort of IH patients. Age at the first visit was approximately 4 months, women were more frequently affected, and most IHs were localized, superficial and located on the trunk and face. We observed that aesthetic impairment was the main reason for starting treatment and that timolol is the most frequent treatment used in daily clinical practice in Spain, while propranolol is reserved for complications or high-risk IH. The characteristics of patients receiving propranolol in a routine clinical setting overlap in many aspects with those of the clinical trial, but there were some differences.
Demographic and clinical characteristics of the AEDV cohortAge at baseline visit in our cohort was similar to several studies published since the first use of propranolol18,23, which was first reported as a treatment for IHs in 200824, but lower than the age reported in studies carried out previously19,25. This finding might reflect the increased training and education of primary care physicians, who have greater accuracy in diagnosis and faster consultation with dermatologists. However, patients may still be seen late by dermatologists, given that most IHs appear at approximately 2 weeks of age and that the fastest growing stage occurs during the second month of life1. Other demographic characteristics, such as sex ratio, weight at birth or gestational age, were similar to those reported in previous studies18,26,27.
The most frequent morphologic subtype was localized, as previously reported28,29. Superficial and mixed IHs, as well as IHs with steep edges, have been related to a higher incidence of sequelae and worse aesthetic outcomes14,15,18. In comparison with these studies, in our cohort, we observed a higher proportion of superficial IHs and a lower proportion of mixed IHs. The prevalence of a cobblestone surface, which has been associated with anetoderma15, was similar between superficial and mixed IHs.
The median size of the IHs in our sample was smaller than that reported by Chamlin et al. in 200712, which could also reflect the earlier detection of and dermatological consultation for IHs. Regarding location, face and trunk were the areas most frequently affected.
The proportion of IH patients in our sample who suffered ulceration or other complications was lower than that reported previously for the use of propranolol12,26. These differences may be due to the effectiveness of the treatment but also to the exclusion of patients who had already started treatment by the time of the baseline visit in our research. Vision was the more frequently impaired function, since a periorbital localization is relatively common16.
Therapeutic approachMore than 50% of patients started treatment at the baseline visit. The rapidly increased use of beta blockers as a treatment for IHs has resulted in a greater proportion of patients who receive treatment than previously reported27. The main reason for starting treatment was aesthetic impairment. Timolol was the most frequent treatment. This drug has a response rate similar to propranolol and higher than laser and observation according to a recent meta-analysis21. However, there are no clinical trials currently assessing its effectiveness, and it is used off-label. The presence of IH complications was an important reason for starting treatment with propranolol, although the main reason, as with timolol, was aesthetic impairment. Female patients and those with segmental IHs (which are associated with ulceration and complications7,12,28), with mixed or deep IHs, with IHs with a larger height and size or with IHs located on the face were more likely to receive propranolol. However, superficial IHs, IHs with a steep edge and IHs with a cobblestone surface, factors related to worse aesthetic outcomes14,15,18, were treated with propranolol less often. A remarkable fact is that 4 of the 5 patients with PHACE syndrome started propranolol, since it is currently considered a safe treatment in the absence of severe arteriopathies30,31. The two patients who did not receive beta-blocker treatment had ulcerated IHs and instead underwent laser treatment.
Comparison with the clinical trialThe use of propranolol is based principally on the outcomes of one clinical trial17, but the restrictive eligibility criteria may limit the generalizability of its results. Eligible patients were 35–150 days old with a proliferating IH requiring systemic therapy with a largest diameter of at least 15 mm. The exclusion criteria comprised, among others, IHs on the diaper area, function-threatening IHs, ulcerated IHs with a lack of response to simple wound care measures, patients who had been previously treated for IH, IHs with clinically uncertain diagnosis (particularly sub-dermal lesions) or patients born preterm who had not yet reached their term-equivalent age.
In our investigation, the age of patients who started propranolol at the baseline visit was similar to that of the patients in the clinical trial, while the female-to-male ratio was higher (3.5:1 vs 2.5:1). Other demographic characteristics were not reported in the trial. Compared with those of the clinical trial, the proportion of localized IHs was lower, that of indeterminate and segmental IHs was higher and that of superficial IHs was similar. Size was not reported in the trial, although the minimum size to meet the inclusion criteria was 15 mm, as mentioned above. In our cohort, only 47 (66%) patients treated with propranolol had an IH diameter larger than 15 mm. The proportion of facial/non-facial involvement was similar. In the clinical trial, the superficial component was only assessed as flat or elevated, and the last category was subdivided into slight, moderate and marked, making it difficult to compare with our results. Data about other IH characteristics, complications and causes of treatment onset were not reported in the clinical trial, which limits data comparison. Therefore, although 1/3 of our patients would not have been included in the trial because of the size of the IH and the proportion of segmental IH (related to worse outcomes) is higher, it seems that propranolol is generally being used in a similar population as that of the trial, but this statement cannot be definitely confirmed due to the lack of data.
Strengths and limitationsThe main strength of this study is that it is one of the largest prospective cohorts of patients with IH following the commercialization of propranolol and is very likely to be representative of the current characteristics and therapy of IHs in the clinic. The main limitation of our study is the exclusion of patients already undergoing treatment at the time of the baseline visit, which could have led to a selection bias. However, as the main outcome measure of the research will be the blindly aesthetic comparison of standardized photographs obtained at the baseline visit prior to treatment onset (or clinical observation) with those obtained at the end of the 5-year follow-up period, the availability of a pre-treatment photograph was considered necessary for inclusion.
ConclusionsIn conclusion, we have presented the baseline characteristics of the Spanish cohort of patients with IHs. Some data, such as age at consultation and size of the IHs, reflect a new scenario for this disease, with physicians better trained on and more concerned with IHs. Approximately 50% of the patients received no treatment. Topical timolol is the most frequent treatment, and aesthetic compromise is the main reason for starting treatment, although there are no clinical trials assessing its outcomes, and it is used off-label. The most important reason for starting propranolol is also aesthetic impairment; another major reason is the presence of complications. Other therapies, such as laser or corticosteroids, are now anecdotal in this age group. Since aesthetic impairment is the main reason for starting treatment, there is a need to better understand the aesthetic results of the different therapies. There is also a need to increase evidence on the use of timolol, which, even if off-label, is currently the most common therapy for IHs. Several characteristics of the patients and IHs treated with propranolol are similar to those of the pivotal clinical trial, even in spite of its restrictive inclusion criteria. However, this statement cannot be definitely confirmed, as 1/3 of IHs in routine clinical practice did not reach the minimum diameter to meet the inclusion criteria of the trial, and important prognostic information, such as size, kind of superficial component, kind of edge, concrete locations, complications or the reasons for starting treatment, were not reported in the original study.
FundingThe study has been funded by the Fundación Piel Sana AEDV.
Conflicts of interestDra. Baselga-Torres reports personal fees and other from Pierre Fabre dermatology, other from Venthera, during the conduct of the study; In addition, Dr. Baselga-Torres has a patent Topical PIK3CA inhibitors for Venous malformations pending. Dra. Vicente reports other from Pierre Fabre, during the conduct of the study; other from Pfizer, other from Lilly, other from Novartis, other from Cellgene, other from Amryt Pharma, other from Sanofi, outside the submitted work. The rest of the authors disclose no conflicts of interest.
We want to than Miguel Ángel Descalzo-Gallego for his valuable contribution to the development of this research.
Please cite this article as: Cuenca-Barrales C, Baselga-Torres E, del Boz-González J, Vicente A, Palencia-Pérez SI, Campos-Domínguez M, et al. Descripción basal de la cohorte nacional prospectiva de hemangiomas infantiles de la Academia Española de Dermatología y Venereología. Comparación de los pacientes tratados con propranolol en la práctica clínica rutinaria y los datos del ensayo clínico pivotal previo. Actas Dermosifiliogr. 2021;112:806–816.