Thiazides are widely used diuretics that first became available in the 1950s. The first reports of photosensitivity reactions to thiazides were published shortly after the introduction of these drugs, but few cases have been described since.
We review all the cases of photosensitivity due to thiazides published up to December 2011. We found 62 cases, 33 in women and 29 in men. The most common presentation was eczematous lesions in a photodistributed pattern, and the most common causative agent was hydrochlorothiazide. The results of photobiological studies were published in only some of the cases reviewed. In most cases, phototesting revealed an abnormal response to UV-A alone or to both UV-A and UV-B. In some cases, the results of phototesting were normal and only photopatch testing yielded abnormal results.
Diagnosis of photosensitivity due to thiazides requires a high degree of suspicion. Ideally, diagnosis should be confirmed by a photobiological study.
Las tiazidas son diuréticos que se comenzaron a usar en la década de 1950 y su uso está muy extendido en la actualidad. Poco después de su introducción se describieron las primeras reacciones de fotosensibilidad, aunque han sido descritas solo de forma infrecuente con posterioridad.
Revisamos los casos de fotosensibilidad por tiazidas publicados hasta diciembre de 2011. Encontramos 62 casos, de los cuales 33 eran mujeres y 29 varones. La forma de presentación más común fue con lesiones eccematosas fotodistribuidas. La hidroclorotiazida fue el agente causal más frecuente. Solo algunos casos publicados recogen el resultado del estudio fotobiológico. En la mayoría el fototest mostró un respuesta alterada a ultravioleta A (UVA) sola y a UVA+ultravioleta B (UVB). En algunos casos el fototest fue normal y solo el fotoparche estaba alterado.
El diagnóstico de fotosensibilidad por tiazidas requiere un alto índice de sospecha. De forma ideal debería confirmarse mediante estudio fotobiológico.
Thiazides have been used in the treatment of arterial hypertension since the late 1950s. This class of drugs includes hydrochlorothiazide, chlorothiazide, chlortalidone, metozalone, bendroflumethiazide, trichlormethiazide, and indapamide. Thiazides achieve an antihypertensive effect by means of direct vasodilatation and inhibit the sodium-chloride cotransporter in the distal convoluted tubule, giving rise to salt and volume depletion.1
The chemical structure of thiazides is derived from that of the sulfonamides, molecules containing a sulfonyl group connected to an amine. This structure is common to many drugs that are otherwise different in terms of structure, molecular weight, and properties. Some sulfonamide-derived drugs, such as dapsone and some oral antidiabetic agents, have photosensitizing potential.2
Drug-induced photosensitivity is determined by the capacity of some medications to modify an individual's sensitivity to solar radiation or artificial light.3,4
Photosensitivity reactions are a growing problem in dermatology. Although new molecules are tested prior to their introduction on the pharmaceutical market, there continue to be new reports of photosensitivity reactions as an adverse effect.3,5
Thiazide diuretics are among the drugs that most frequently cause photosensitivity reactions.5 The prevalence of clinical photosensitivity in patients receiving treatment with thiazides is estimated at between 1 and 100 per 100 000 patients.6 However, despite being widely used, thiazides have received little attention in the literature.
The first thiazide-induced photosensitivity reactions were reported shortly after the introduction of these drugs.7,8 Hydrochlorothiazide, the most commonly used thiazide, is implicated in most cases of thiazide-induced photosensitivity.6 Other clinical manifestations of thiazide-induced photosensitivity are vasculitis,9 lichenoid reactions, and erythema multiforme.10
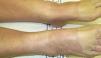
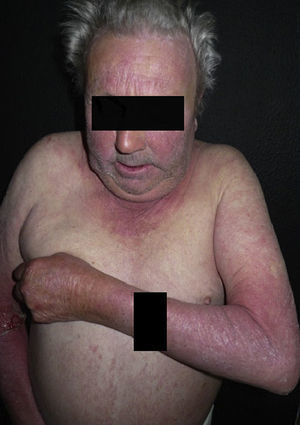
Clinical ManifestationsSystemic thiazide-induced photosensitivity reactions present clinically as dermatoses with a symmetrical distribution in sun-exposed areas with localized lesions on the face, the upper chest, the dorsal aspect of the forearms, and the hands (Fig. 1). There are usually well-defined borders between the affected sun-exposed areas and the areas covered by clothing, jewelry, glasses, watches, etc. (Fig. 2).3,10,11 However, disseminated lesions have also been reported.10
The following clinical manifestations of thiazide-induced photosensitivity have been reported to date:
- a)
Erythema. Clinically very similar to the erythema of sunburn. Patients may report burning and/or itching sensations, in some cases very intense.10
- b)
Eczema. Scaly, erythematous plaques with an eczematous appearance.12
- c)
Subacute cutaneous lupus erythematosus (SCLE)–like eruptions. Scaly, erythematous plaques that are clinically and histologically indistinguishable from idiopathic SCLE. In addition, anti-Ro/SS-A and anti-La/SS-B antinuclear antibodies may be present in the serum and immune complex deposition may be present in the basement membrane.10,13–18
- d)
Lichenoid reaction. Clinically and histologically similar to lichen planus.7,19
- e)
Photodistributed petechial reaction.8
- f)
Pseudoporphyria. Clinically and histologically similar to porphyria cutanea tarda, but without accompanying porphyrin metabolism abnormalities.20,21
- g)
Photoonycholysis. Usually accompanied by a generalized cutaneous photosensitivity reaction; less frequently, it appears as the sole manifestation of photosensitivity.22
- h)
Pigmentation. Usually occurs after an erythematous reaction and generally presents as diffuse hyperpigmentation in sun-exposed areas, although cases with a reticular pattern have also been reported. The reaction subsides as clinical photosensitivity improves, although slight hyperpigmentation persists in some cases.10
- i)
Persistent photosensitivity. Robinson et al.23 described 4 patients with what they termed chronic photosensitivity [sic], which they attributed to hydrochlorothiazide ingestion. In some cases, the involvement of the drug was questionable. Addo et al.10 raised doubts regarding the conclusions of Robinson et al., noting that they had never encountered a case of thiazide-induced persistent photosensitivity.
- j)
Cheilitis. One case has been reported in which cheilitis of the lower lip was the only manifestation of photosensitivity. The authors of the case report did not carry out any photobiologic tests but considered that the lesions could be attributed to a photosensitivity reaction induced by metformin or hydrochlorothiazide.24
- k)
Carcinogenesis. A slightly increased risk of cutaneous squamous cell carcinoma and melanoma has been found in patients receiving combined amiloride and hydrochlorothiazide therapy.25
- l)
Photoleukomelanoderma. Brownish erythematous macules with hypochromic areas that appear on sun-exposed skin.26
To date, 62 cases of photosensitivity attributed to thiazides have been reported (Tables 1–4).
Isolated Cases of Eczematous Thiazide-Induced Photosensitivity Reactions.
| Author(s) | Age, y | Sex | Drug | Time Since Onset | Time Since Introduction of the Drug | Clinical Manifestations | Biopsy | ANAs | Photopatch Testing | Phototesting |
| Harber et al.7 (1959) | 60 | Woman | Chlorothiazide | NA | NA | Acute eczematous reaction | No | NA | No | Yes (with natural light after suspension of treatment) |
| White11 (1983) | 68 | Man | HCTZ | 5 wk | 3 mo | Acute eczematous reaction | No | NA | No | Yes |
| Schwarze et al.12 (1998) | 70 | Woman | Altizide | NA | 15 y | Erythematous papulosquamous eruption | No | NA | Yes: UV-A (−), UV-B (+) | Yes: UV-A and UV-B (−) |
Abbreviations: ANAs, antinuclear antibodies; HCTZ, hydrochlorothiazide; NA, not available.
Cases of Thiazide-Induced Subacute Cutaneous Lupus Erythematosus.
| Author(s) | No. of Cases | Age, y | Sex | Drug | Time Since Onset | Time Since Introduction of the Drug | Clinical Manifestations | Biopsy | ANAs | Photobiologic Testing |
| Reed et al.14 (1985) | 5 | 42-68 | 4 men, 1 woman | HCTZ | 1-10 mo | 6 mo-5 y | SCLE | Yes | + in 1 case, − in 4 cases | No |
| Addo et al.10 (1986) | 1 | 64 | Woman | HCTZ | NA | NA | SCLE | Yes | − | Yes |
| Darken et al.15 (1988) | 3 | 61-75 | 2 men, 1 woman | HCTZ | 1.5-3 mo | NA | SCLE | Yes | + in 1 case, − in 3 cases | No |
| Parodi et al.16 (1989) | 1 | 64 | Woman | HCTZ | 2 mo | 4 mo | SCLE | Yes | − | No |
| Brown et al.17 (1995) | 1 | 65 | Man | HCTZ | NA | 2 y | SCLE | Yes | + | No |
| Srivastava et al.18 (2003) | 5 | 44-65 | 4 men, 1 woman | HCTZ | NA | 1-2 mo | Photodistributed erythema in all cases, SCLE in 2 cases | Yes | + in all cases | No |
Abbreviations: ANAs, antinuclear antibodies; HCTZ, hydrochlorothiazide; NA, not available; SCLE, subacute cutaneous lupus erythematosus.
Atypical Forms of Thiazide-Induced Photosensitivity.
| Author(s) | No. of Cases | Age, y | Sex | Drug | Time Since Onset | Time Since Introduction of the Drug | Clinical Manifestations | Biopsy | ANAs | Photobiologic Testing |
| Norins8 (1958) | 1 | 87 | Woman | HCTZ | NA | 2 wk | Petechiae | No | No | No |
| Harber et al.7 (1959) | 2 | 66-71 | Women | HCTZ, chloro-thiazide | NA | NA | Lichen planus | Yes | NA | Yes |
| Robinson et al.23 (1985) | 4 | 44-68 | 2 men, 2 women | HCTZ | 1-20 y | NA in 3 cases, 2 y in 1 case | Eczematous chronic persistent photosensitivity | Yes | − in 3 cases, NA in 1 case | Yes (only after withdrawal of the drug in 3 cases) |
| Motley20 (1990) | 1 | 65 | Man | HCTZ | 12 mo | 1 mo | Pseudoporphyria in areas affected by vitiligo | NA | NA | No |
| Johnston19 (2002) | 1 | 77 | Man | HCTZ | 2 mo | 6-8 mo | Lichen planus | Yes | − | No |
| Masuoka et al.26 (2011) | 1 | 68 | Man | HCTZ | 6 mo | 9 mo | Photoleukomelanoderma | Yes | NA | Yes |
Abbreviations: ANAs, antinuclear antibodies; HCTZ, hydrochlorothiazide; NA, not available.
Cases of Thiazide-Induced Photosensitivity Studied by Addo et al.
| Addo et al. studied 33 patients with photosensitivity reactions who had been taking thiazides; the manifestations were attributed to the diuretic in 24 cases. |
| Age: 29-75 y |
| Clinical manifestations:Most patients presented variable combinations of erythema, edema, desquamation, and pigmentation.An SCLE–like eruption was present in 1 patient. |
| Time since onset of manifestations and duration of treatment: NA. |
| Phototesting: Abnormal response inUV-A+UV-B: 10 patientsUV-A: 11 patientsUV-A+visible light: 2 patientsUV-A+UV-B+visible light: 2 patientsUV-B: 1 patient |
| Photopatch testing: Not performed |
| Immunologic studies: NA in most cases. Ambiguous result for ANAs in 1 patient. |
| Histologic studies: Performed in 5 patients. Showed features of spongiotic dermatitis. |
| Clinical course and treatment: Thiazide was withdrawn in 16-18 patients. Treatment was maintained and sun protection measures were introduced in 6 patients. |
Abbreviations: ANAs, antinuclear antibodies; NA, not available; SCLE, subacute cutaneous lupus erythematosus.
Of these patients, 29 were men and 33 were women. Hydrochlorothiazide was implicated in most cases. However, many of the patients had also been taking other drugs (in some cases unspecified).
Interestingly, SCLE-like eruptions were present in 15 cases (Table 2), lichenoid or lichen planus–like reactions in 3 cases, and pseudoporphyria in 2 cases (Table 3), probably because infrequent reactions are reported more frequently.
The largest case series of thiazide-induced photosensitivity was described by Addo et al.10 (Table 4). The study included 33 cases of patients with photosensitivity reactions. In 24 cases, the reaction was attributed to a thiazide. In the remaining cases, it was more difficult to confirm the involvement of a thiazide because the patients were also taking other photosensitizing drugs or another photodermatosis was present.
Photobiologic TestingOnly a few of the cases of thiazide-induced photosensitivity reported to date include phototest results.7,10–12,23,26 Addo et al.10 carried out photobiologic tests using a monochromator and a solar simulator (Table 4). Abnormal responses were found in the UV-A and UV-B ranges in 10 patients; in the UV-A range in 11 patients; in the UV-A, UV-B, and visible ranges in 2 patients; in the UV-A and visible ranges in 2 patients; and in the UV-B range in 1 patient. In 10 patients, a phototest was carried out after the suspected drug was withdrawn to determine baseline values. The response had returned to normal in 7 patients. In the 3 patients in whom the abnormal response persisted, either the treatment had been suspended recently or another cause was present. The time to normalization of the phototest results was variable. In most cases, the results of a follow-up phototest at 2 months were normal. However, the time elapsed before normalization is unknown because the test was performed when the patient returned to the clinic; the precise moment between the 2 tests at which the response returned to normal is not known. The time to resolution of clinical photosensitivity ranged from 1 to 6 months. None of the patients developed chronic actinic dermatitis.
Masuoka et al.26 reported the case of a Japanese man who had an abnormal reaction to UV-A radiation while taking hydrochlorothiazide and losartan; the treatment was suspended and the reaction had resolved at 2 months. A positive result was obtained in a UV-A photopatch test. Schwarze et al.12 reported a case of photosensitivity in a patient who had been receiving treatment with altizide and spironolactone. Phototest results were normal, but a UV-B photopatch test was positive and a UV-A photopatch test was negative. Both of these patients had been taking drugs that combined a thiazide with another active ingredient. In both cases, the photosensitivity reaction was attributed to the thiazide. However, the photopatch tests were performed with the combination of drugs rather than with each drug separately. Neither article specified whether photopatch tests were performed in controls.
Robinson et al.23 reported 4 cases of patients with persistent photosensitivity possibly related to prior treatment with hydrochlorothiazide. Phototesting revealed alterations in all 4 cases, but in 3 cases the tests were performed after the treatment had been discontinued. The minimal erythema dose (MED) for UV-B was lower than normal in 2 patients, normal in 1 patient, and was not obtained for the fourth patient. The reaction to UV-A radiation was abnormal in 3 patients and normal in 1 patient. None of the patients underwent photopatch testing. The authors attributed the patients’ photosensitivity to their earlier ingestion of thiazides. Addo et al.10 raised doubts about the conclusions of this study, noting that they had never encountered a case of thiazide-induced persistent photosensitivity.
Molecular MechanismsAlthough thiazide-induced photosensitivity is well known, the mechanism of action is poorly understood.27 However, damage to membrane lipids and DNA has been demonstrated.27–29
Various techniques have been used to predict the photosensitizing potential of drugs, including in vitro studies (photohemolysis test, lipid peroxidation test, Candida albicans test, cell line studies, and others),30 studies in animal models,5,31 and studies in humans.6,32
Because no test has been able to detect all possible photosensitizing agents, it is important that multiple photosensitivity screening methods be used.27
UV-A radiation appears to be responsible for the photosensitizing effects of sulfonamide-derived drugs.5 However, in vivo and in vitro studies have indicated that UV-B and UV-A radiation could have additive or even synergistic effects.33
Management of Photosensitivity ReactionsDiagnosisA suspected diagnosis is established on the basis of physical examination and medical history. Ideally, the diagnosis should be confirmed by photobiologic tests.
If the distribution of the lesions suggests a photosensitivity reaction, the physician should obtain a directed medical history that includes any drugs the patient is taking, the date of the start of treatment, and any changes in dose. The physician should also ask about the patient's history of sun exposure and outdoor activities. Additionally, histopathologic studies should be performed in order to determine the inflammatory response pattern and a differential diagnosis with other entities should be established.
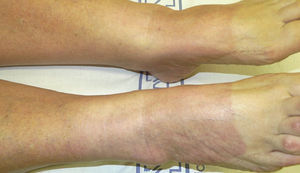
PhototestingPhototests should be performed while the patient is still taking the drug in order to establish the UV-B MED and the response to UV-A radiation (Fig. 3).10 The phototest should be repeated 3 weeks after the suspected drug is withdrawn. If the MED remains low or the reaction to UV-A radiation persists, the phototest should be repeated every 2 to 3 months until results are normal. There is no consensus on how long should be allowed for the results to return to normal, but in our department we allow 1 year. If a patient's phototest results have not changed 1 year after the withdrawal of the drug, we consider that the results are normal for that patient. Results are considered to be normal when the MED has increased by 40% with respect to the previous test or when the reaction to UV-A radiation is no longer present.34 One limitation of the phototest is that there is no well-defined normal value for the UV-B MED. Therefore, there is no way to know a priori whether a UV-B MED value is normal or low. Follow-up assessments and additional phototests enable the physician to determine whether the UV-B MED value was normal or low for a particular patient.
Phototest in a patient with hydrochlorothiazide-induced photosensitivity. The upper set was exposed to UV-A radiation with a UV 181 AL lamp and the lower set was exposed to UV-B radiation with a solar simulator. An abnormal reaction to UV-A radiation and low UV-B minimal erythema dose can be observed.
Photobiologic testing is relatively simple in patients who are being treated with a single drug. However, it is fairly common to encounter patients who are taking multiple drugs. In these patients, the drugs must be withdrawn one at a time—starting with known photosensitizing agents—and a phototest must be performed after each successive withdrawal. We have occasionally seen patients who report medical histories suggestive of drug-induced photosensitivity but have already stopped taking the suspected drug by the time they visit our department. In such cases, we cannot carry out a phototest while the patient is still taking the drug (unless we decide to reintroduce it for this purpose). Moreover, we cannot know whether the patient's MED without the drug is at least 40% higher than his/her MED while taking the drug. In such cases, it is only possible to establish a presumptive diagnosis on the basis of clinical improvement.
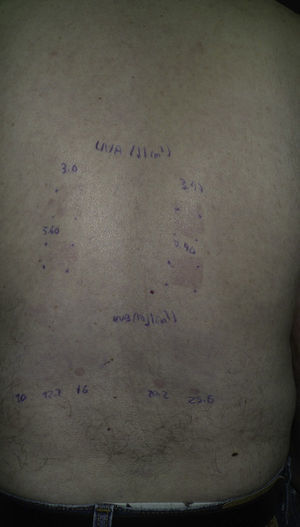
Photopatch TestingIn photopatch testing, photoallergens are applied in duplicate on unaffected skin to the patient's back. After 48hours, one of the sets is irradiated with UV-A radiation at a dose of 5 J/cm2.35,36 The test results are read at 24 to 48hours after irradiation, although subsequent readings can also be made. A positive photopatch test result is necessary in order to confirm a diagnosis of contact photoallergy. In systemic photosensitivity, however, the photopatch test is often negative. A positive result confirms the diagnosis of systemic photosensitivity induced by the tested drug, but a negative result does not rule out this diagnosis. A negative result can occur when photosensitivity is caused by metabolites in the drug rather than the drug itself. Because most photoallergic reactions have been attributed to UV-A radiation, it is therefore generally recommended that photopatch tests be carried out with UV-A radiation. However, there have been cases of systemic photosensitivity in which photopatch testing obtained a positive result with a sub-MED dose of UV-B radiation while showing no response to UV-A radiation.12,36
TreatmentWhen a patient has an adverse reaction to a drug, withdrawal of treatment is usually recommended. If the medication is essential and no valid therapeutic alternative is available, the type and severity of the adverse reaction should be assessed before the medication is withdrawn definitively. If the medication is not essential, the presence of a clinical photosensitivity reaction justifies its withdrawal. Sun protection measures are useful in the initial stage of a photosensitivity reaction. Antihistamines and corticosteroids can also be used as symptomatic treatment if the reaction is intense. Psoralen–UV-A therapy has been used successfully in cases of persistent photosensitivity possibly induced by thiazides.23 The introduction of sun protection measures can allow patients to continue treatment.10
It is important to remember that thiazides can cross-react with sulfonamides, paraphenylenediamine,37 and para-aminobenzoic acid, which is found in some sunscreens.4
A loop diuretic with a lower photosensitizing potential (such as bumetanide) could be used as an alternative to hydrochlorothiazide.3 Although furosemide and bumetanide are chemically related to sulfonamides, only very rarely do they present hypersensitivity cross-reactions with thiazides.
Another way to reduce clinical photosensitivity could be to switch to nighttime administration of the medication, as is done with other photosensitizing drugs such as quinolones. Nighttime administration could be useful in the case of thiazides but no studies on this approach have been published. However, for diuretics such as hydrochlorothiazide, which reaches its maximum effect 4hours after ingestion, nighttime administration may not be appropriate.
ConclusionsThiazides are a widely used class of drugs whose safety profile is generally good. Photosensitivity reactions are the most frequent cutaneous adverse effects of these drugs. Thiazide-induced photosensitivity reactions can have different clinical patterns, with eczematous reactions being the most frequent. If possible, the diagnosis should be confirmed by phototesting and photopatch testing. Once a thiazide has been identified as the causative agent in a photosensitivity reaction, the definitive treatment is to withdraw the drug and/or introduce sun protection measures. Dermatologists must therefore be familiar with the diagnosis and management of these reactions.
Ethical DisclosuresProtection of persons and animalsThe authors declare that no experiments were performed on humans or animals for the purpose of this study.
Data confidentialityThe authors declare that they have followed the protocols of their hospitals concerning the publication of patient data and that all patients included in this study were appropriately informed and gave their written informed consent.
Right to privacy and informed consentThe authors declare that no patient data are disclosed in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Gómez-Bernal S, Álvarez-Pérez A, Rodríguez-Pazos L, Gutiérrez-González E, Rodríguez-Granados MT, Toribio J. Fotosensibilidad por tiazidas. Actas Dermosifiliogr. 2014;105:359–366.