Hand eczema affects nearly 10% of the population. The condition becomes severe and chronic in 5% to 7% of cases and is refractory to topical corticosteroids in 2% to 4%. This study aimed to describe the current use of oral alitretinoin in treating Spanish national health system patients with hand eczema that is refractory to potent topical corticosteroids.
Materials and methodsObservational, descriptive, exploratory, cross-sectional study based on the retrospective analysis of records for patients with hand eczema treated with alitretinoin in the Spanish national health system.
ResultsWe reviewed the records for 62 patients in 13 hospitals in 5 different administrative areas (autonomous communities) of Spain. Alitretinoin was usually used at a dosage of 30mg/d. In most cases the physician judged the clinical response to be satisfactory after a single cycle. The recorded adverse effects were foreseeable and of the type reported for systemic retinoids. The dermatologists agreed that the clinical benefits achieved with alitretinoin favored adherence to treatment and an early return to work.
ConclusionsThe results show that oral alitretinoin is being used according to established recommendations and that response is good, with few adverse effects. The dermatologists agreed that the benefits favored adherence and improved the patients’ health related quality of life.
El eczema de manos (ECM) afecta a cerca del 10% de la población, presentándose entre el 5 y el 7% de los casos como una enfermedad crónica grave y siendo refractario al tratamiento con corticoides tópicos entre el 2 y el 4% de las veces. El propósito del artículo es describir el uso de la alitretinoína oral en pacientes con ECM refractario a corticoides tópicos potentes en el ámbito sanitario público español.
Material y métodosEstudio observacional descriptivo, exploratorio, transversal, basado en la revisión retrospectiva de historias clínicas de pacientes con ECM en tratamiento con alitretinoína en el ámbito sanitario público español.
ResultadosSe revisaron 62 historias clínicas de pacientes de 13 centros distribuidos en 5 comunidades autónomas del territorio español. Alitretinoína se utilizó predominantemente a dosis de 30mg/día, principalmente en un único ciclo, tras el cual la mayoría de pacientes lograron una respuesta clínica satisfactoria según el juicio médico. Los eventos adversos fueron todos previsibles y en línea con los tratamientos sistémicos con retinoides. Los dermatólogos estuvieron de acuerdo en que los beneficios clínicos logrados con alitretinoína favorecían la adherencia al tratamiento y una reincorporación más rápida de los pacientes al trabajo.
ConclusiónLos resultados muestran un uso de alitretinoína oral en línea con las recomendaciones establecidas así como la buena respuesta al tratamiento asociado y los pocos efectos adversos. Los dermatólogos coinciden que los beneficios alcanzados favorecen la adherencia al tratamiento y mejoran la calidad de vida relacionada con la salud de los pacientes.
Chronic hand eczema (CHE) is hand eczema that lasts for over 3 months or that occurs 2 or 3 times in a period of 12 months, despite treatment.1 It is characterized by erythema, vesicles, papules, cracks, hyperkeratosis, pruritus, and pain, and symptoms can range from mild to disabling.2,3 Hand eczema affects approximately 10% of the population4,5; between 5% and 7% of these have severe chronic disease, while 2% to 4% have disease that does not respond to treatment with topical corticosteroids.1
In Spain, an estimated 5000 people have severe, refractory hand eczema, and most of these are working-age adults.6,7 CHE can cause work-related difficulties and may also be associated with social stigma, leading to possible rejection and impaired patient quality of life.8,9
Severe CHE is treated with potent topical corticosteroids.1 Other treatment possibilities are topical calcineurin inhibitors, phototherapy with psoralens and long-wave UV radiation (PUVA), and systemic treatments with corticosteroids or immunosuppressants, such as ciclosporin.10–12
Alitretinoin is the only oral treatment currently approved by the Spanish Agency of Medicines and Medical Devices (AEMPS) for use in severe CHE that is unresponsive to treatment with potent topical corticosteroids.13 Alitretinoin is an intracellular retinoid receptor pan-agonist. It regulates cell multiplication, differentiation, and apoptosis, and also has immunomodulatory and anti-inflammatory effects that are relevant to skin inflammation.14 Its efficacy and safety in the treatment of severe CHE have been evaluated in adult patients administered daily doses of 10 to 30mg over 12 to 24 weeks.4,15–17
The main aim of this study was to describe the use of alitretinoin to treat CHE within the Spanish public health system. We provide a detailed description of the sociodemographic and clinical characteristics of the patients treated, their response and adherence to treatment, their health-related quality of life, and the overall level of satisfaction with the drug among prescribing physicians.
MethodsDesignWe conducted a descriptive, observational, exploratory, cross-sectional study consisting of a chart review of patients with CHE treated with alitretinoin within the Spanish public health system between July and September 2012.
Study PopulationAt the time of the study, alitretinoin was only available through hospitals, specialized dermatology units, and hospital pharmacies following authorization from the AEMPS. All patients treated with alitretinoin during the study period were identified from medical records. We included patients aged over 18 years with a diagnosis of CHE who had been treated with alitretinoin and whose medical records contained information pertaining to at least 1 follow-up visit at the time of data collection. We excluded patients with a diagnosis other than CHE involving skin infections or other skin disorders, of any etiology, that could interfere with the evaluation of response to alitretinoin. The patients were selected and included in the study sequentially, not randomly.
Twenty-one public hospitals were invited to participate in the study.
Study VariablesUsing a purpose-designed case report form, we recorded various sociodemographic variables (age, sex, job status and sector, and contact with substances associated with CHE) and the following clinical variables: associated concomitant diseases (e.g., atopic dermatitis, allergy, asthma, and rhinoconjunctivitis); personal history of disease (diabetes, thyroid disease, liver failure, kidney failure, hypercholesterolemia, hypertriglyceridemia, hypervitaminosis A, hypertension, obesity, and other dermatologic or systemic diseases); date of diagnosis and diagnostic tests performed; type, location, and severity of CHE; associated signs and symptoms; dose, frequency, and duration of alitretinoin treatment; previous treatments for CHE; concomitant treatments unrelated to CHE and concomitant treatments for CHE; occurrence and severity of adverse events; treatment response assessments by the treating physician (including Physician Global Assessment, Patient Global Assessment, and modified Total Lesion Symptom Score18); treatment response; laboratory tests ordered during diagnosis and follow-up; results of these tests; and pregnancy prevention plan where relevant.
To evaluate physician satisfaction with response to treatment, coinciding with the data collection phase of the study, the participating physicians were asked to complete a purpose-designed questionnaire (available as supplementary material) regarding their satisfaction with the use of alitretinoin in the study population, the type of clinical response obtained, and the level of treatment adherence and compliance. The questionnaire also contained items related to satisfaction with prescribing conditions and adherence to recommendations for the use of alitretinoin. The questions were answered using a 5-point Likert-type scale (1=not at all satisfied, 2=not very satisfied, 3=satisfied, 4=quite satisfied, and 5=very satisfied).
Ethical RequirementsThe retrospective design of the study required the participating hospitals to have a policy in place that met the conditions of confidentiality and nontraceability under the Spanish Data Protection Law. The study protocol was approved by the clinical research ethics committee at Hospital Puerta de Hierro, in Madrid, Spain.
ResultsParticipating Hospitals and Number of Medical Records ReviewedOf the 21 hospitals approached, 13 agreed to participate in the study; they were located in 5 of Spain's autonomous communities: Andalusia (n=2), Aragon (n=1), Castilla-la-Mancha (n=2), Catalonia (n=2), and Madrid (n=7). Seventy-two relevant medical records were identified, and 62 (86%) of these met the inclusion criteria. The reasons for exclusion were a change in diagnosis from CHE to psoriasis during treatment (n=1), treatment for psoriasis (n=1), diagnosis of chronic eczema with foot involvement only (n=2), no clinical history (n=1), and no clinical follow-up (n=5).
Sociodemographic CharacteristicsTable 1 summarizes the patients’ sociodemographic characteristics and employment situation. The mean (SD) age of the patients was 53 (13) years, and there was a similar proportion of men (46.8%) and women (53.2%). Approximately 40.3% of patients (n=25) were in employment at the time of the study; 9.7% were homemakers, 9.7% were pensioners, and 1.6% were unemployed. Most patients were working in the textile, chemical, or plastics industries (11.3%), followed by the domestic work sector (9.7%), the construction sector (8.1%), and the hotel and catering industry (8.1%). Two patients mentioned that CHE had been a determining factor for changing employment, and 6 mentioned it as a reason for temporarily not being able to work.
Sociodemographic Characteristics of Patients With Chronic Hand Eczema (CHE) Treated With Alitretinoin.
| Characteristics | |
|---|---|
| Mean (SD) age, y | 52.73 (12.97) |
| Male sex, % | 46.8 |
| Currently in employment, % | 40.3 |
| Change of work sector following diagnosis of CHE, % | 3.2 |
| Inability to work due to CHE, % | 9.7 |
| Contact with substances that cause CHE, % | 41.9 |
Tests were used to diagnose CHE in 75.8% of patients (n=47). The mean (SD) number of tests was 1.7 (0.6) and the most common tests were patch tests (66.1%; n=41) and biopsy (29.0%; n=18).
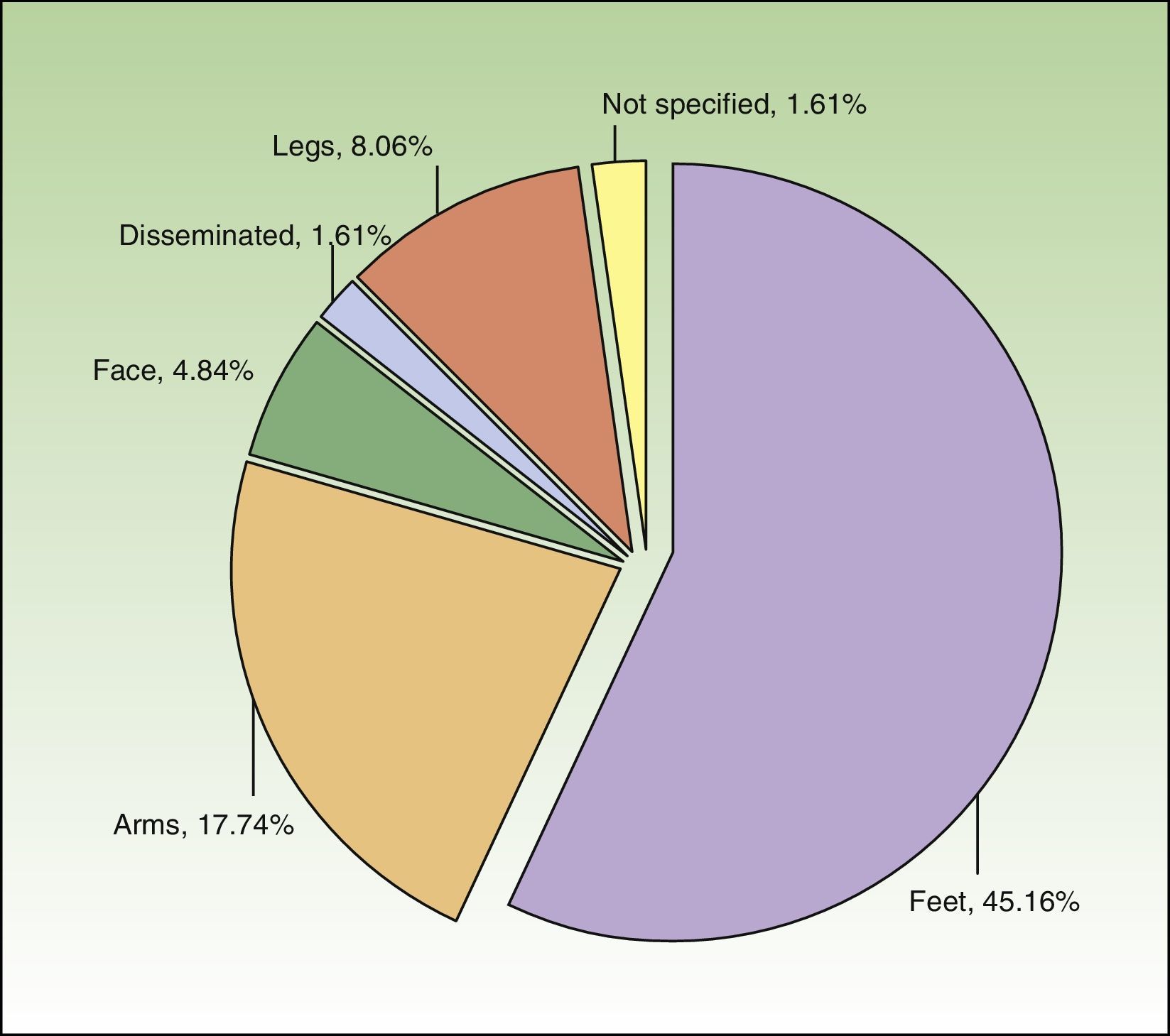
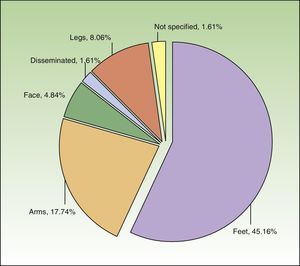
The most common subtype of CHE was hyperkeratotic fissured hand eczema (29.0%; n=18). Eight patients (12.9%) had more than 1 subtype. CHE was observed most frequently on the palms and backs of the hands (69.35%, n=43 and 16.13%, n=10, respectively). Just over two-thirds of patients (n=42) had hand involvement only, but 19.35% (n=12) and 11.29% (n=7) had involvement of 2 and 3 sites, respectively. Figure 1 shows the additional sites affected.
At least 1 symptom was recorded for 62.9% of patients (n=39). Symptoms included pruritus (61.3%), pain (16.1%), and burning (3.2%). Pruritus was mild in 2 cases, moderate in 3, and intense in 17. Severity of involvement was not specified in the remaining 16 cases. Pain, in turn, was moderate in 2 cases (16.1%), intense in 4, and not specified in another 4. Finally, burning was described as moderate in 2 patients (3.2%). In total, 90.3% of patients (n=56) had clinical manifestations of CHE, namely reddening, flaking, vesicles, edema, and cracks. The most common signs were flaking (62.9%, n=39), reddening (45.2%, n=28), and cracks (38.7%, n=24).
Severity of CHE at the time of diagnosis was noted for 69.3% of patients. The intensity of the eczema was recorded using different terms (e.g., severe, intense, considerable), which were considered equivalent for our analysis. Nine patients were reported to have severe CHE, while 21 were considered to have moderate CHE (identified by the terms outbreak, moderate, and moderate to intense).
There was mention of nonresponse to potent topical corticosteroids in 87.1% of the medical records (n=54). This lack of response was identified by terms such as no response (69.4%, n=43) and corticosteroid-dependent (9.7%, n=6). Impact on quality of life was mentioned, together with treatment resistance, in 5 cases (8.1%). There was no mention of the use of recommended tools to evaluate these characteristics of CHE.
Over half of the patients’ records (58.1%; n=36) mentioned 1 or more concomitant diseases associated with CHE. The most common ones were atopic dermatitis (22.6%, n=14), allergy (16.1%, n=10), and rhinitis (9.7%, n=6).
Prior Treatment to AlitretinoinThe patients in our series received a mean (SD) of 3 (1.6) medications before being prescribed alitretinoin. Topical treatments included corticosteroids (83.9%, n=52), immunomodulators (33.9%, n=21), and topical retinoids (25.8%, n=16). Phototherapy was used in 16.1% of cases (n=10).
Over half of the patients (56.4%, n=35) received systemic treatment for CHE at some point of their disease. Systemic therapies included oral corticosteroids (43.5%, n=27), immunosuppressants (33.9%, n=21), analgesics, and antibiotics (1.6%, n=1). Antihistamines had been prescribed to 14 patients (22.6%) and antigout agents (e.g., colchicine) and antimalarials had each been prescribed to 3 patients (4.8%).
Treatment With AlitretinoinOf the 62 patients included in the study, 83.9% (n=52) received a single cycle of alitretinoin, 14.5% (n=9) received 2 cycles, and just 1.6% (n=1) received 3 cycles.
The mean (SD) time between treatment cycles was 6.7 (8.9) months for the first and second cycle and 16 months for the second and third cycle. Alitretinoin 30mg/d was used in 83.8% of patients (n=52) during the first cycle and in 66.7% of patients (n=6) during the second one. The patient who received a third cycle was also prescribed alitretinoin 30mg/d for this last cycle. The initial dose of 30mg was reduced to 10mg in 1 patient (1.6%) due to headache, and in another patient, an initial dose of 10mg was increased to 30mg on day 20. During the second cycle, the approved dose was changed to a 30-mg dose every 48hours in 2 patients (22.2%). The median duration of treatment was 15.9 weeks (range, 2-82 weeks) for the first cycle and 8.3 weeks (range, 4-65 weeks) for the second cycle. Duration of treatment in the only third cycle administered was 65 days.
The median time from onset of alitretinoin treatment to the first evaluation of response was 35 days (range, 0-607 days) for the first cycle and 42 days (range, 0-61 days) for the second cycle. Time to evaluation was 90 days for the patient who received the third treatment cycle.
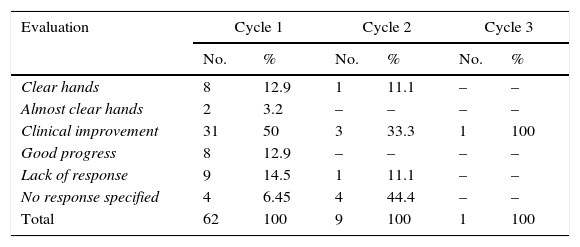
Use of a recommended tool for assessing treatment response was recorded in 35.5% of cases. Table 2 summarizes the results per cycle. In most cases, response to treatment (clear or almost clear hands) was based on the physician's clinical assessment.
Distribution of Sample According to Evaluation of Response to Oral Alitretinoin After Each Cycle.
| Evaluation | Cycle 1 | Cycle 2 | Cycle 3 | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Clear hands | 8 | 12.9 | 1 | 11.1 | – | – |
| Almost clear hands | 2 | 3.2 | – | – | – | – |
| Clinical improvement | 31 | 50 | 3 | 33.3 | 1 | 100 |
| Good progress | 8 | 12.9 | – | – | – | – |
| Lack of response | 9 | 14.5 | 1 | 11.1 | – | – |
| No response specified | 4 | 6.45 | 4 | 44.4 | – | – |
| Total | 62 | 100 | 9 | 100 | 1 | 100 |
Considering the data recorded for treatment response, an improvement in CHE was reported for 49 patients (79%) (10 of whom achieved clear or almost clear hands), while no response was reported for 9 (14.5%). There was no mention of response in 4 cases (6.4%). Improvement was observed in 4 (44.4%) of the 9 patients who received a second cycle of treatment. For this cycle, there was 1 nonresponder (11.1%) and no mention of response in 4 cases (44.4%). Clinical improvement was observed at the first follow-up visit for the patient who underwent the third cycle of alitretinoin.
Approximately 43.5% of patients (n=27) were prescribed a concomitant topical treatment, namely emollients (22.6%, n=14), high-potent corticosteroids (19.4%, n=12), and potent corticosteroids (3.2%, n=2). Four patients (6.5%) were coadministered systemic corticosteroids.
Safety MonitoringAlmost all of the patients’ medical records (98.4%) included information on laboratory tests used to monitor the safety of alitretinoin treatment. A median of 2 tests (range, 0-13 tests) was performed per patient. Most patients (93.4%) showed biochemical alterations during the different treatment cycles. The most common alterations were lipid profile alterations (hypercholesterolemia, 32.3%; hypertriglyceridemia, 3.2%; and hypercholesterolemia and hypertriglyceridemia, 6.5%), followed by changes in white blood cell count (3.2%), elevated thyroid stimulating hormone levels (3.2%), and hyperglycemia (1.6%). None of these alterations led to an interruption of treatment.
Pregnancy Prevention PlansOf the 33 women studied, 20 (60.6%) were of reproductive age and therefore should have been following a pregnancy prevention plan during alitretinoin treatment. There was, however, no mention of contraceptive methods in the records of 70.0% (n=14) of these patients. Of the remaining 6 women, 4 had been advised to take oral contraceptives. The other 2 did not require a pregnancy prevention plan, as 1 had had a hysterectomy and the other was with a partner who had undergone a vasectomy.
SafetyThere was no mention of adverse events in 18 (29%) of the 62 patients studied. Seventeen patients (27.4%) did not experience any adverse effects, and 27 (43.5%) experienced at least 1 adverse effect associated with alitretinoin. The most common effect was headache (25.8%), followed by xerophthalmia and xerosis (4.8% in both cases).
Alitretinoin treatment was interrupted in 19 patients. The reasons for interruption were a loss of response (n=5), worsening of CHE (n=2), and adverse effects (headache and swelling at night) (n=2).
Physician Satisfaction With Alitretinoin TreatmentAll the dermatologists surveyed were satisfied with treatment effectiveness and duration, type of response and duration, and safety and tolerability. They all mentioned that they would recommend alitretinoin for the treatment of CHE.
Almost all of the dermatologists (91.7%) were satisfied, quite satisfied, or very satisfied with the percentage of responders observed in their practice. The same proportion also noted that treatment adherence or compliance (defined as “the extent to which a patient acts in accordance with the prescribed interval and dose of a dosing regimen”19) was better with alitretinoin than with other treatments prescribed for CHE. In addition, 83.3% observed better adherence to alitretinoin treatment than with the most common treatments used in CHE. Seventy-five percent of dermatologists considered that patients treated with alitretinoin had been able to return to work sooner than if they had received other treatments. Two-thirds of the dermatologists surveyed stated that they were not satisfied with the dispensing and prescribing conditions in place at the time of the study (compassionate use).
DiscussionThe World Health Organization (WHO) encourages studies that investigate the use of medication in routine clinical practice.18 According to the WHO, over 50% of drugs are prescribed, dispensed, or sold inadequately, and in addition, over 50% of patients fail to take drugs correctly. Inappropriate use of medicines has direct repercussions in terms of undesirable adverse effects, poor results, and inappropriate use of health care resources. In this study, we have described how alitretinoin is routinely used in clinical practice by dermatologists with experience in both the treatment of CHE and the use of oral retinoids. Our findings show that alitretinoin is not entirely used in accordance with the recommendations in the summary of product characteristics.14 However, the compilation of data was complicated by the fact that the participating hospitals used different documentation criteria for their medical records. Compliance with recommendations in the medical literature on how to document treatment response was also suboptimal considering that response is a key factor in the decision regarding whether or not to continue treatment.9
Despite the limitations inherent to the design of our study, our results show good response to treatment with alitretinoin and acceptable tolerability. Our findings for treatment effectiveness and safety, patient characteristics, and clinical manifestations of CHE coincide those reported by a prospective, descriptive, observational study performed in Spain.4 That study reported very similar complete and partial response rates to ours (clear and almost clear hands) and also described a very similar adverse event profile, with a predominance of headache. Our study differs in only 1 aspect, which was that the most common subtype of CHE in our series was hyperkeratotic fissured hand eczema (29.03%). Our findings, however, are limited by the heterogeneous definitions and recording of criteria related to disease severity, chronicity, concomitant treatments, and resistance to previous treatments.
It should be noted that both the use of alitretinoin and the clinical information available at the time of the study were marked by a degree of uncertainty. Such uncertainty is typical in the context of recurrent and disabling disorders, such as CHE, for which alternative treatments are limited, frequently used off-label, and associated with undetermined clinical response. Alitretinoin, however, differs in this respect, as its use in severe, refractory CHE is approved and supported by clinical response data.15–17
Because severe, refractory CHE is relatively uncommon in Spain and access to alitretinoin is restricted, there were relatively few candidates for inclusion in this study. Nonetheless, our findings are important as they describe how a specific drug is routinely used to treat a disease in the Spanish public health care system, although our results cannot be extrapolated to other health care areas.
The data compiled show trends in usage and the need to standardize the documentation of patient information and the use of tools for evaluating clinical response and treatment goals. Our findings for the levels of satisfaction among dermatologists using alitretinoin should also be interpreted within the context of the number of physicians who answered the questionnaire.
In conclusion, alitretinoin is used to treat severe, refractory CHE, is mainly used at a dose of 30mg/d administered in a single cycle, and achieves satisfactory clinical response in the opinion of the treating physicians. The adverse events observed were all predictable and consistent with those seen in systemic retinoid therapy. Finally, the dermatologists surveyed all agreed that the clinical benefits associated with alitretinoin favored treatment adherence and a speedier return to work.
Ethical DisclosuresProtection of humans and animalsThe authors declare that no tests were carried out in humans or animals for the purpose of this study.
Confidentiality of dataThe authors declare that no private patient data appear in this article.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
FundingThis study was sponsored by Almirall, SA.
Conflicts of InterestDr S Urrutia and Dr G Roustan report having received fees for contributing their expert knowledge to the topic of interest. S Paz and L Lizán work for an independent research entity and have received fees for contributing to the development and coordination of the original research project and for preparing an initial draft of this manuscript. All fees were paid by the study sponsor, Amirall SA. Dr MJ Plazas and Dr S Armengol work at Amirall SA.
Nonetheless, the authors declare that the results of the research described in this article, together with the analysis and interpretation of the result, are based on the free opinion and consensus of the authors, and that no conflicts arose in relation to the production or dissemination of these results.
The authors would like to thank Stiefel/GSK for checking the accuracy of the product information reported in this article.
Please cite this article as: Urrutia S, Roustan G, Plazas MJ, Armengol S, Paz S, Lizan L. Uso de la alitretinoína oral para el tratamiento del eczema crónico de manos grave y refractario al tratamiento en el sistema sanitario público español: descripción y análisis de la práctica clínica actual. Actas Dermosifiliogr. 2016;107:142–148.