Multiple cutaneous and uterine leiomyomatosis (MCUL), or Reed syndrome, is characterized by the presence of cutaneous leiomyomas arising from the arrector pili muscles and, in women, by uterine leiomyomas. In some cases, MCUL is associated with renal cell carcinoma. This syndrome is an autosomal dominant disorder caused by a heterozygous germline mutation of the gene that encodes fumarate hydratase, a Krebs cycle enzyme that acts as a tumor suppressor.
ObjectiveTo review the cases of MCUL diagnosed at 2 university hospitals over a 5-year period (2008-2013).
Material and methodsThis was a retrospective study of 13 cases of MCUL that investigated demographic, clinical, and histologic characteristics, as well as possible associations with other diseases and treatments received.
ResultsWe identified 13 patients (10 women and 3 men) who had been diagnosed with MCUL. The mean age at diagnosis was 53 years. All the patients had multiple cutaneous leiomyomas; in 12 (92%) the distribution was clustered and 9 (69%) also had disseminated solitary lesions. In 1 patient (7.7%), the pattern of distribution was linear. Uterine fibroids requiring hysterectomy were present in 90% of the women. Nine patients were screened for renal lesions; no cases of renal cell carcinoma were detected but benign renal lesions were found in 4 patients.
ConclusionsThe clinical and histologic characteristics of the 13 cases of MCUL reviewed were similar to those reported in the literature. The most common cutaneous manifestation was a type 2 segmental pattern. It is important for dermatologists to identify cutaneous leiomyomas and be aware of the possible association with MCUL.
La leiomiomatosis cutánea y uterina múltiple (MCUL) o síndrome de Reed se caracteriza por la presencia de leiomiomas cutáneos de origen pilar, leiomiomas uterinos en las mujeres y, en algunos casos, asociación con carcinoma renal. Este síndrome, de herencia autosómica dominante, se produce por una mutación heterocigótica en la línea germinal del gen de la fumarato hidratasa, una enzima del ciclo de Krebs que actúa como supresor tumoral.
ObjetivoRevisar los casos de MCUL diagnosticados en 2 hospitales universitarios durante un periodo de 5 años (2008-2013).
Material y métodosEstudio retrospectivo de 13 casos de MCUL, en el que se recogieron características demográficas, clínicas e histológicas, así como posibles asociaciones con otras enfermedades y tratamientos recibidos.
ResultadosTrece pacientes fueron diagnosticados de MCUL (10 mujeres y 3 hombres, con una edad media al diagnóstico de 53 años). El 100% de los casos presentaba leiomiomas cutáneos múltiples, distribuidos de forma difusa (69%), agrupada (92%) y/o lineal (7,7%). El 90% de las mujeres presentaba además miomas uterinos y todas ellas habían precisado histerectomía por ese motivo. No encontramos ningún caso de carcinoma renal en los pacientes explorados (9/13), pero sí lesiones renales benignas (4/9).
ConclusiónDescribimos 13 casos de MCUL, que presentan características clínicas e histológicas similares a las descritas en la literatura, siendo la manifestación cutánea más frecuente la segmentaria tipo 2. Es importante que el dermatólogo identifique los casos de leiomiomas cutáneos y conozca su posible relación con MCUL.
Multiple cutaneous and uterine leiomyomatosis (MCUL), which is also known as multiple leiomyomatosis, Reed syndrome, and leiomyomatosis cutis et uteri, was described by Reed and colleagues in 1973. The syndrome is characterized by cutaneous leiomyomas in men and cutaneous leiomyomas and uterine fibroids in women. There is also a variant associated with renal cell carcinoma known as hereditary leiomyomatosis and renal cell carcinoma (HLRCC).1
Both MCUL and HLRCC are caused by a heterozygous mutation in the fumarate hydratase gene, FH, located on chromosome 1q42.3-q43.2,3
The aim of this study was to describe our experience with MCUL and discuss the characteristics of this syndrome, as well as associated diseases and treatments.
Material and MethodsWe analyzed patients diagnosed with MCUL at the dermatology departments of Hospital Universitari Sagrat Cor and Hospital del Mar in Barcelona, Spain, between 2008 and 2013. We included patients with 10 or more lesions that were clinically consistent with multiple cutaneous leiomyomas and at least 1 lesion with histologic confirmation.
We analyzed demographic, clinical, and histologic characteristics and personal and family history of uterine fibroids, renal lesions, and renal cancer. The data were obtained from the patients’ medical records and some patients were asked to return to the hospital for reevaluation of lesions and completion of medical history.
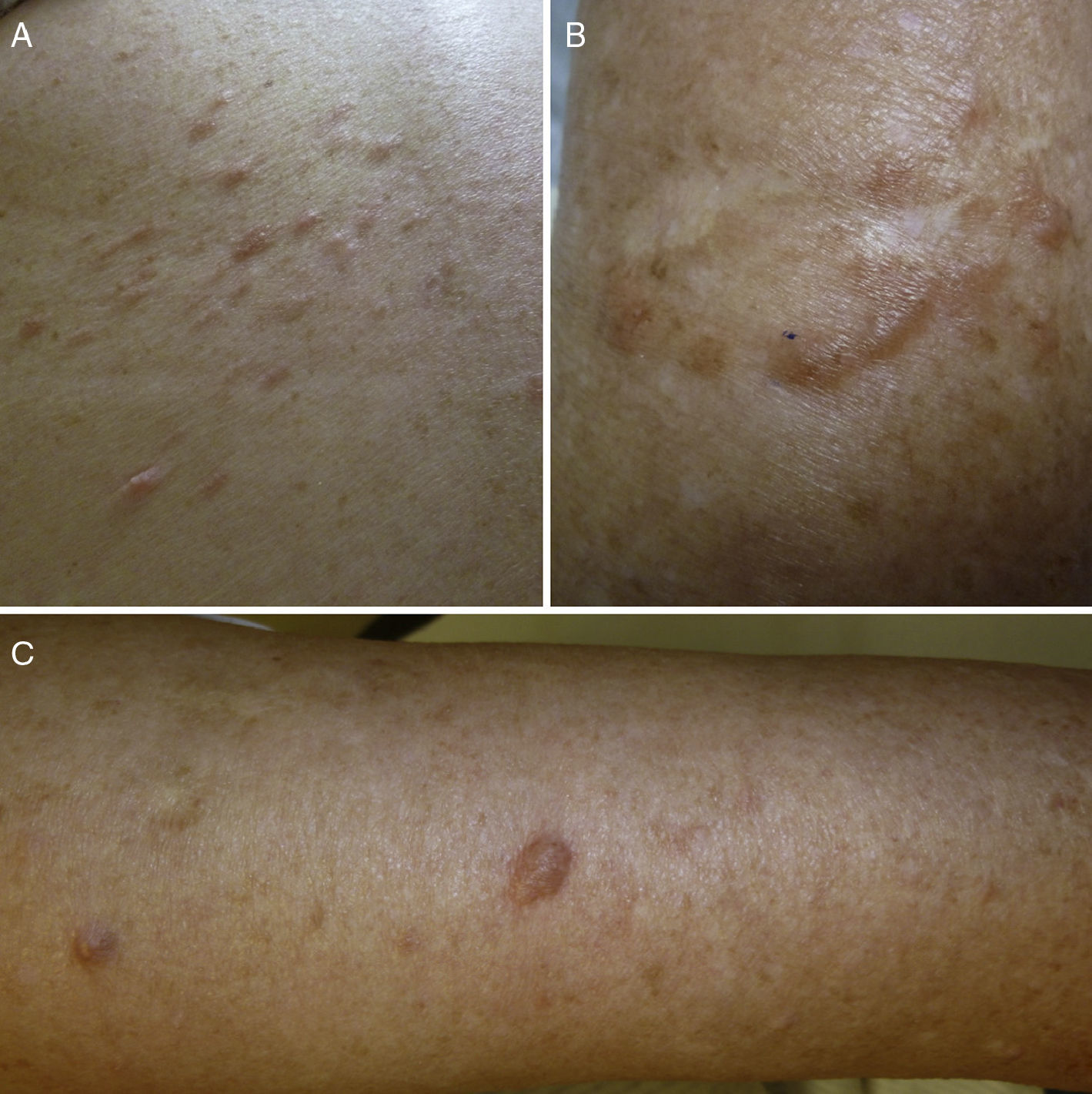
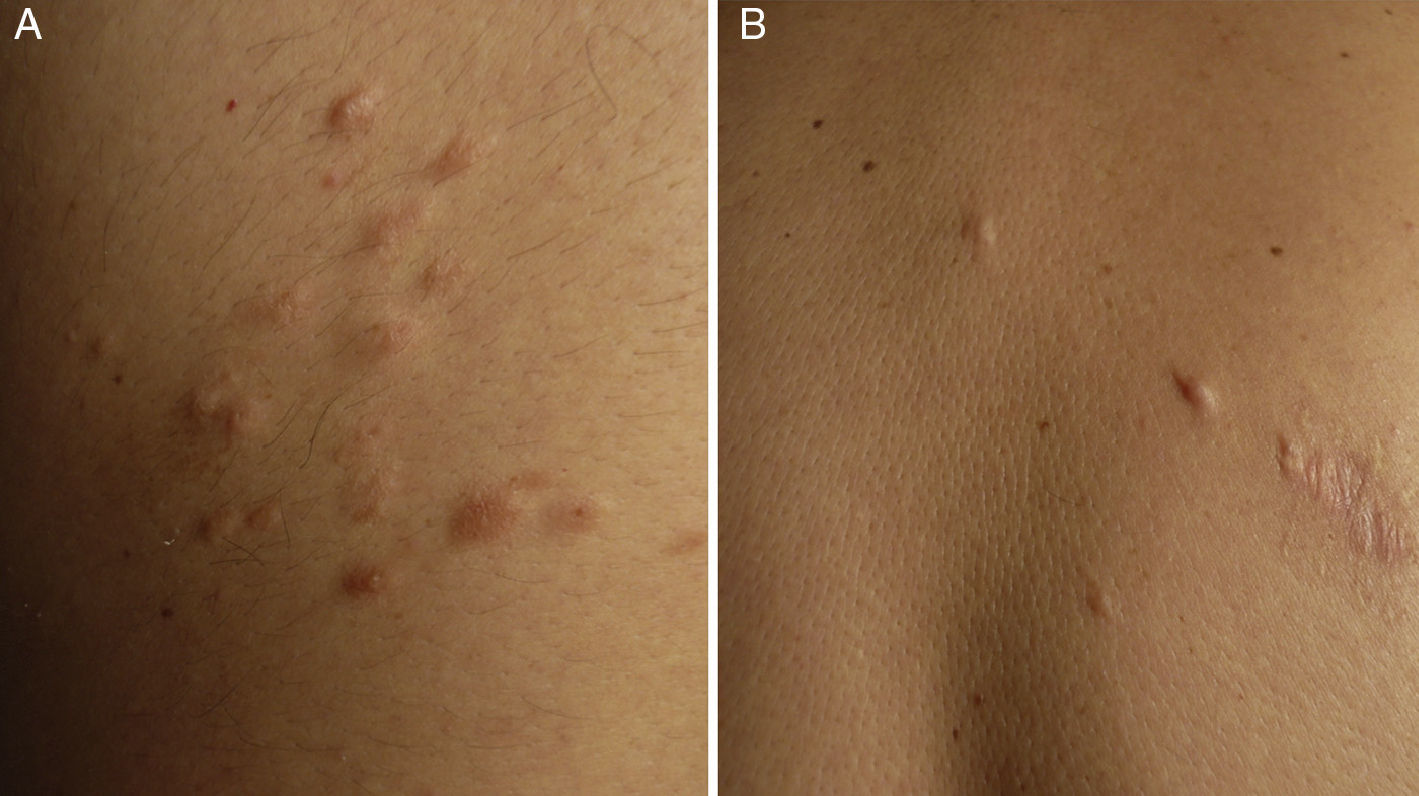
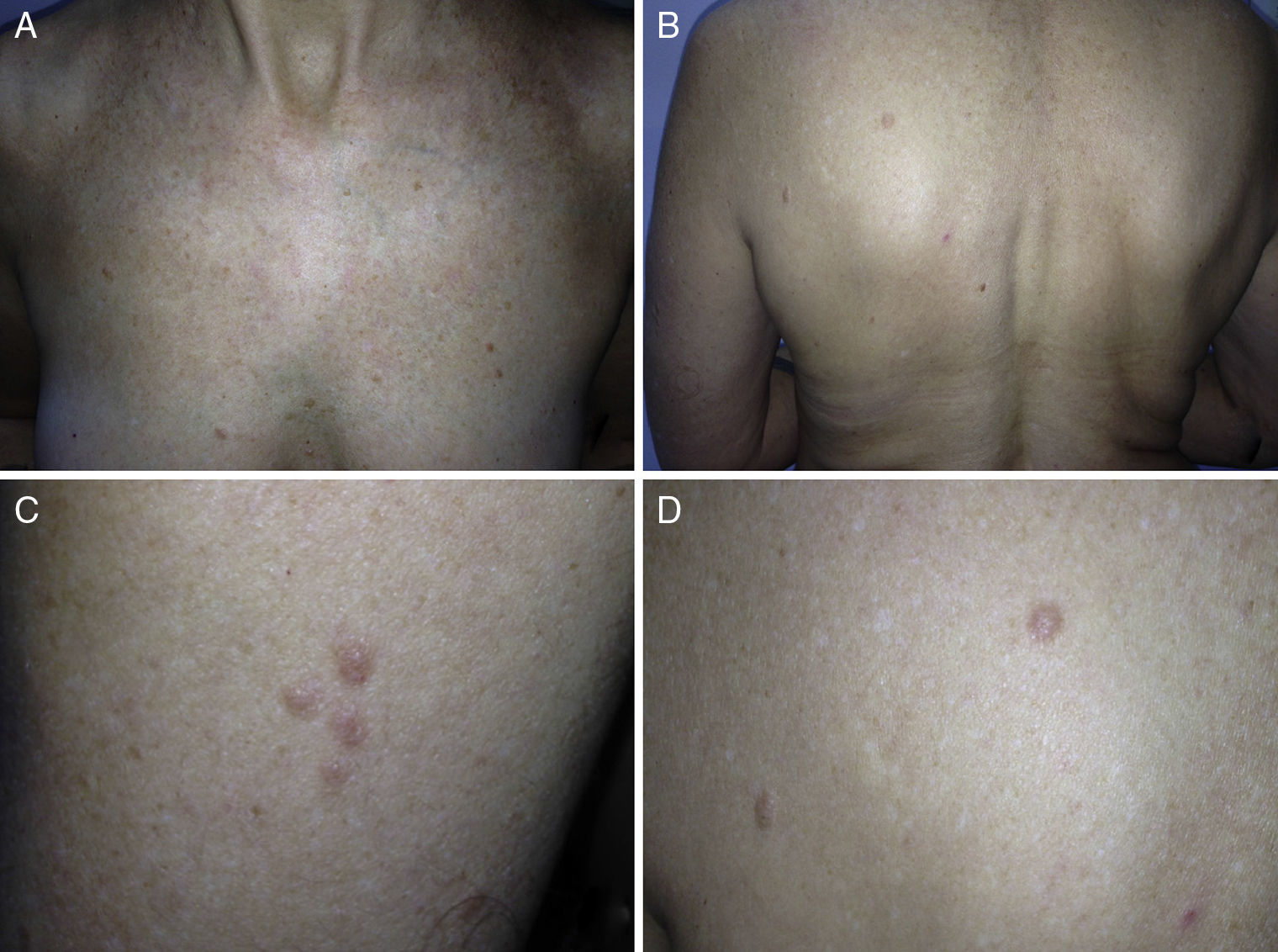
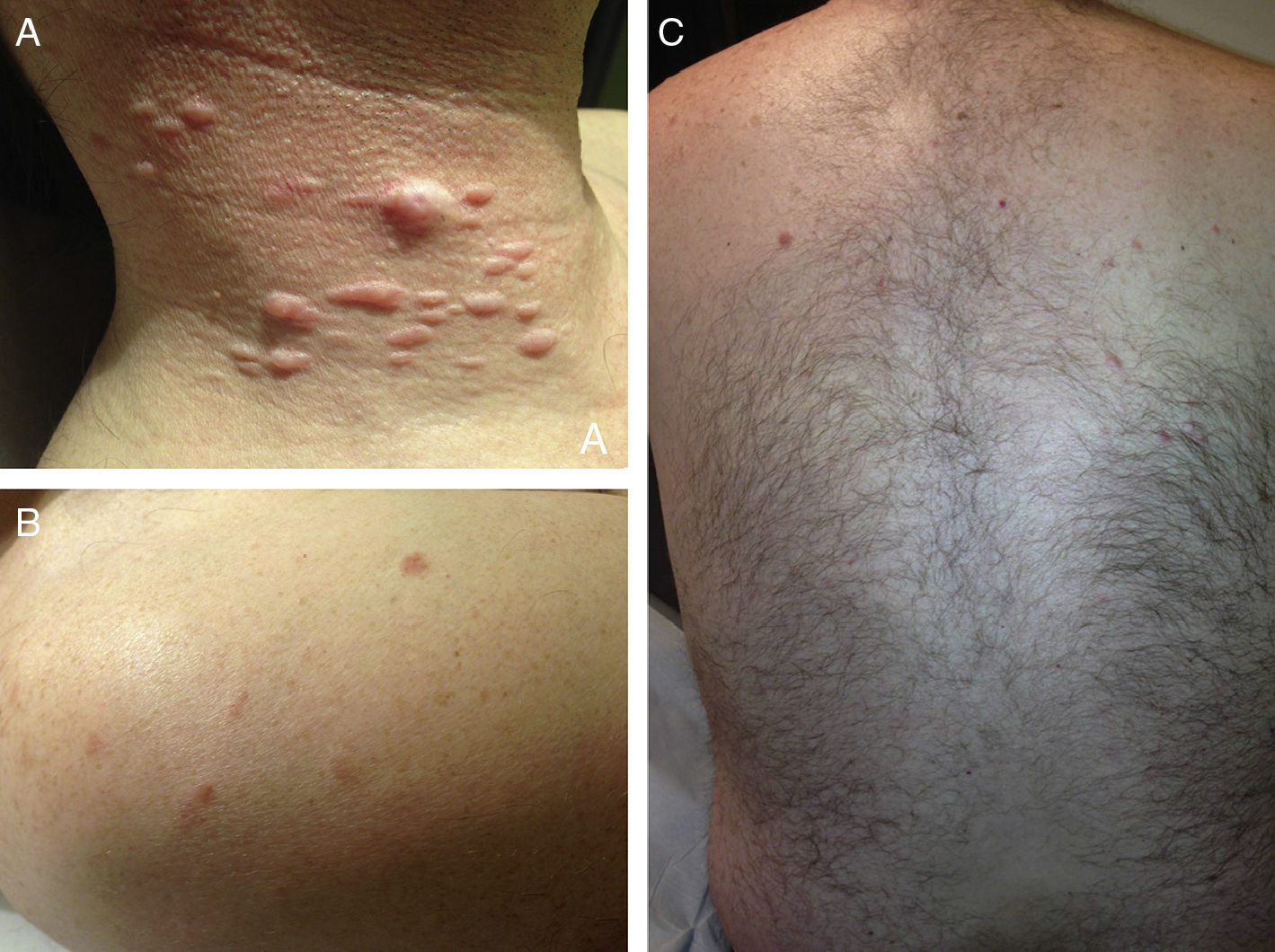
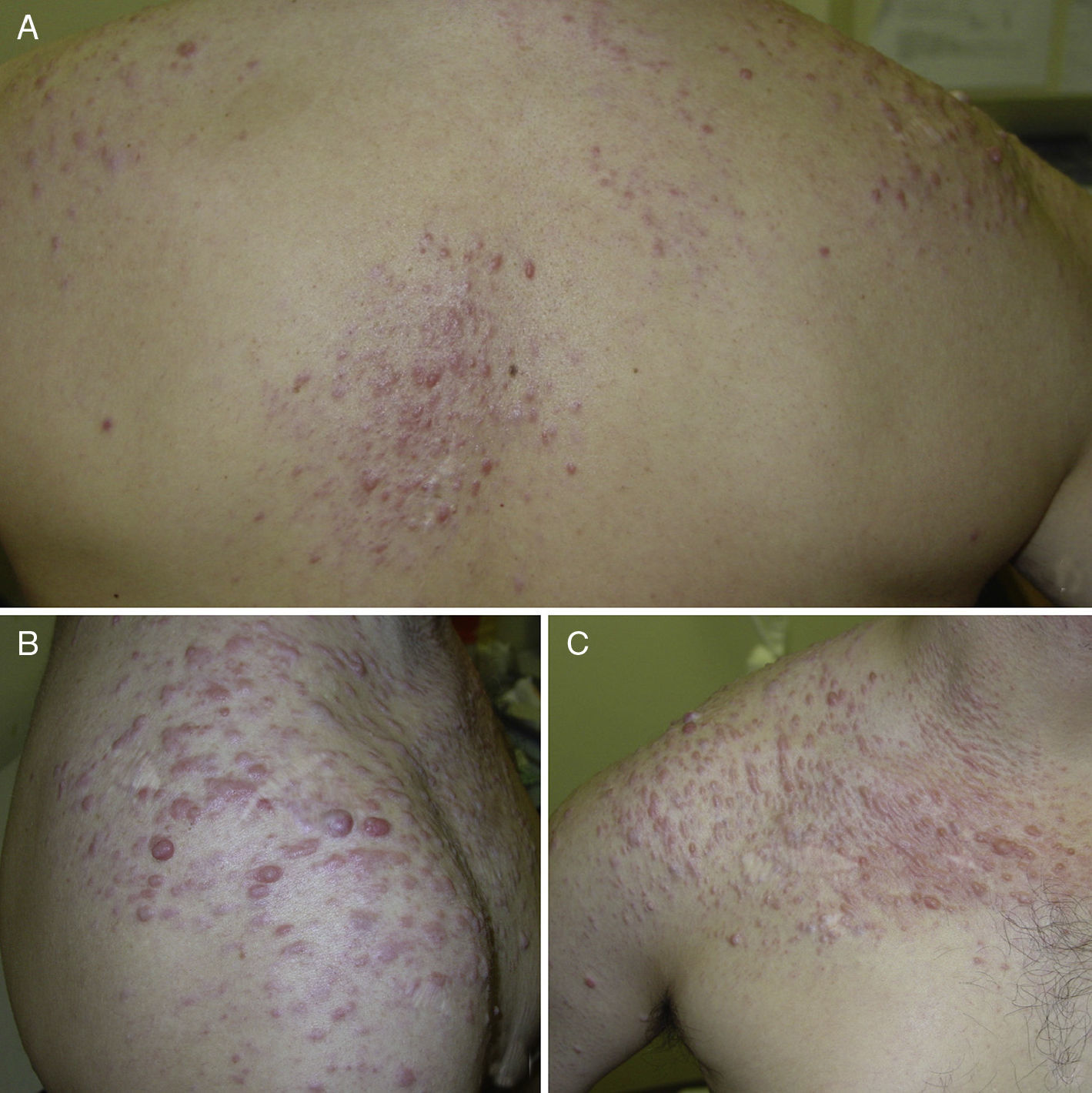
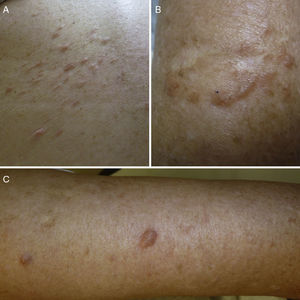
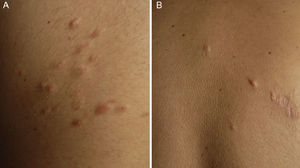
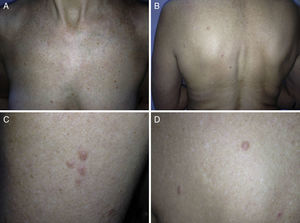
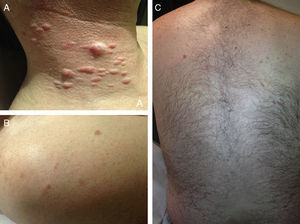
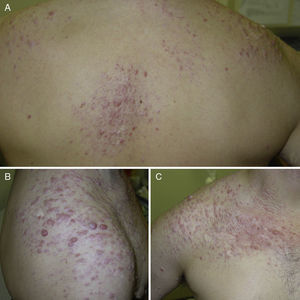
ResultsWe identified 13 patients (10 women and 3 men) with multiple cutaneous leiomyomas. The mean age at diagnosis was 53 years (range, 36-76 years). All the patients had cutaneous lesions and these had been the reason for consultation in 92% of cases (12 of 13 patients). The mean age of onset was 40 years (range, 14-53 years), although this information was not available for 4 patients. There were groups of lesions forming plaques in 12 patients, 9 of whom also had diffuse, isolated lesions. The plaques were located in the chest area in 5 cases, on the neck in 4 cases, on the back in 3 cases, on the upper limb in 3 cases, and on the abdomen in 1 case. The isolated lesions were located on the trunk and the upper and lower limbs (Figs. 1-4). Patient #7 had extensive disease, with multiple plaques on the trunk (Fig. 5). One patient had lesions in a linear pattern limited to the area of the deltoid muscle. Five patients reported pain; this was constant in 2 patients and exacerbated by cold temperatures in another. A sixth patient reported discomfort and occasional itching, and the rest of the patients were asymptomatic.
The most common histologic subtype was piloleiomyoma (in 12 of 13 patients), and there was 1 case of vascular leiomyoma. Cells were positive for actin and desmin in the 2 patients in whom immunohistochemical stains were used.
Nine (90%) of the 10 women had uterine fibroids and they had all required hysterectomy prior to the diagnosis of MCUL.
Screening for renal lesions was performed in 9 of the 13 patients; 4 had renal lesions (cysts, calculi, or adrenal nodule). No evidence of renal cell carcinoma was detected. Imaging studies to rule out renal involvement were not performed in 4 patients, but they were all asymptomatic.
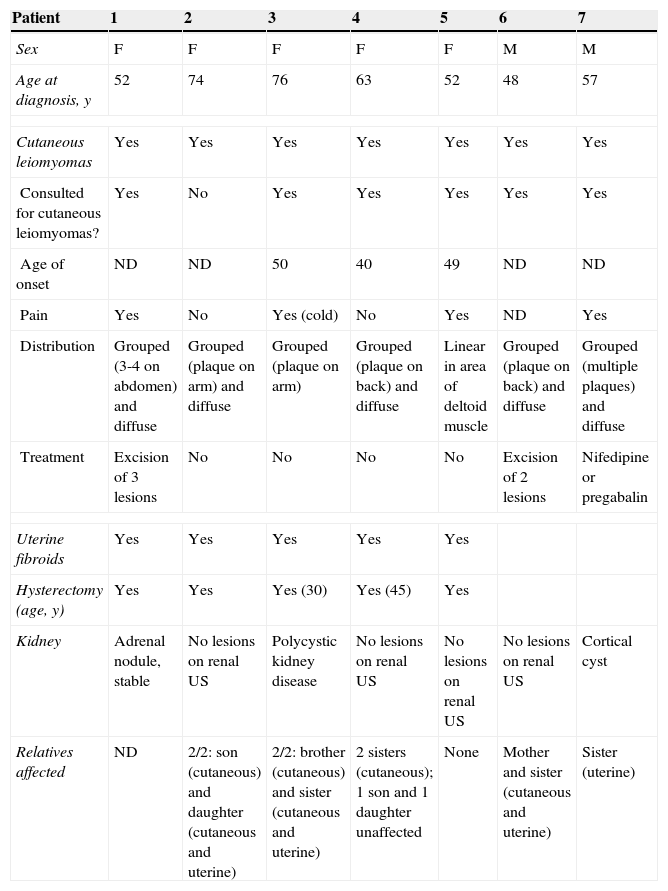
On reviewing associations with other diseases, we found 1 case of hypogonadotropic hypogonadism (in patient #7), but we believe the association was coincidental. Family history was investigated in 12 patients, 11 (92%) of whom had at least 1 relative with cutaneous leiomyomas and/or uterine fibroids. There was no history of renal cell carcinoma, but 1 first-degree relative had died of cancer of unknown origin at the age of 35 years. Four (30%) of the 13 patients did not require treatment. Five patients (38%) had between 1 and 3 lesions excised for clinical or cosmetic reasons, 4 patients (30%) were treated with cryotherapy, with little or no success, and 2 patients (15%) were treated with electrocoagulation or carbon dioxide (CO2) ablation. The 2 patients with the most extensive involvement required multiple treatments for symptomatic relief, including systemic drugs such as nifedipine, pregabalin, doxazosin, and gabapentin. Improvement, however, was minimal. The patients’ characteristics are summarized in Table 1.
Demographic and Clinical Characteristics and Treatment in 13 Patients With Multiple Cutaneous and Uterine Leiomyomatosis.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Sex | F | F | F | F | F | M | M |
| Age at diagnosis, y | 52 | 74 | 76 | 63 | 52 | 48 | 57 |
| Cutaneous leiomyomas | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Consulted for cutaneous leiomyomas? | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Age of onset | ND | ND | 50 | 40 | 49 | ND | ND |
| Pain | Yes | No | Yes (cold) | No | Yes | ND | Yes |
| Distribution | Grouped (3-4 on abdomen) and diffuse | Grouped (plaque on arm) and diffuse | Grouped (plaque on arm) | Grouped (plaque on back) and diffuse | Linear in area of deltoid muscle | Grouped (plaque on back) and diffuse | Grouped (multiple plaques) and diffuse |
| Treatment | Excision of 3 lesions | No | No | No | No | Excision of 2 lesions | Nifedipine or pregabalin |
| Uterine fibroids | Yes | Yes | Yes | Yes | Yes | ||
| Hysterectomy (age, y) | Yes | Yes | Yes (30) | Yes (45) | Yes | ||
| Kidney | Adrenal nodule, stable | No lesions on renal US | Polycystic kidney disease | No lesions on renal US | No lesions on renal US | No lesions on renal US | Cortical cyst |
| Relatives affected | ND | 2/2: son (cutaneous) and daughter (cutaneous and uterine) | 2/2: brother (cutaneous) and sister (cutaneous and uterine) | 2 sisters (cutaneous); 1 son and 1 daughter unaffected | None | Mother and sister (cutaneous and uterine) | Sister (uterine) |
| Patient | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|
| Sex | F | F | F | F | F | M |
| Age at diagnosis, y | 48 | 51 | 37 | 55 | 36 | 40 |
| Cutaneous leiomyomas | Yes | Yes | Yes | Yes | Yes | Yes |
| Consulted for cutaneous leiomyomas? | Yes | Yes | Yes | Yes | Yes | Yes |
| Age of onset, y | 42 | 47 | 35 | 53 | 33 | 14 |
| Pain | No (discomfort and occasional itching) | No | No | No | No | Yes (constant pain from neck lesions) |
| Distribution | Grouped (plaque on arm) and diffuse (back and neckline) | Grouped (plaque in left chest area) | Grouped (plaque on chest and back) and diffuse (neck) | Grouped (neck and left chest area) and diffuse (neck) | Grouped (right chest area) | Grouped (plaque in right laterocervical area) and diffuse (trunk and limbs) |
| Treatment | Excision of 1 lesion | Excision of 1 lesionLiquid nitrogenElectrocoagulation | Liquid nitrogen | Liquid nitrogen | Liquid nitrogen | Excision of 2 lesions, carbon dioxide laser, doxazosin, oral gabapentin |
| Uterine fibroidsHysterectomy | YesYes | YesYes | NoNo | YesYes | YesYes | |
| Kidney | Not examined | Calculi (left) | Pending renal USCystitis | No lesions on renal US | Not examined | Not examined |
| Relatives affected | Grandmother (cutaneous and hysterectomy), mother (died of cancer of unknown origin at age 35), 3 children unaffected | 1 sister (cutaneous and uterine) | 1 sister (uterine) | 1 daughter (hysterectomy) | 1 sister (cutaneous and uterine) and 2 nieces (uterine) | Father, paternal grandmother (cutaneous), paternal aunt (cutaneous), 1 daughter without lesions |
Abbreviations: F, female; M, male; ND, not described; US, ultrasound.
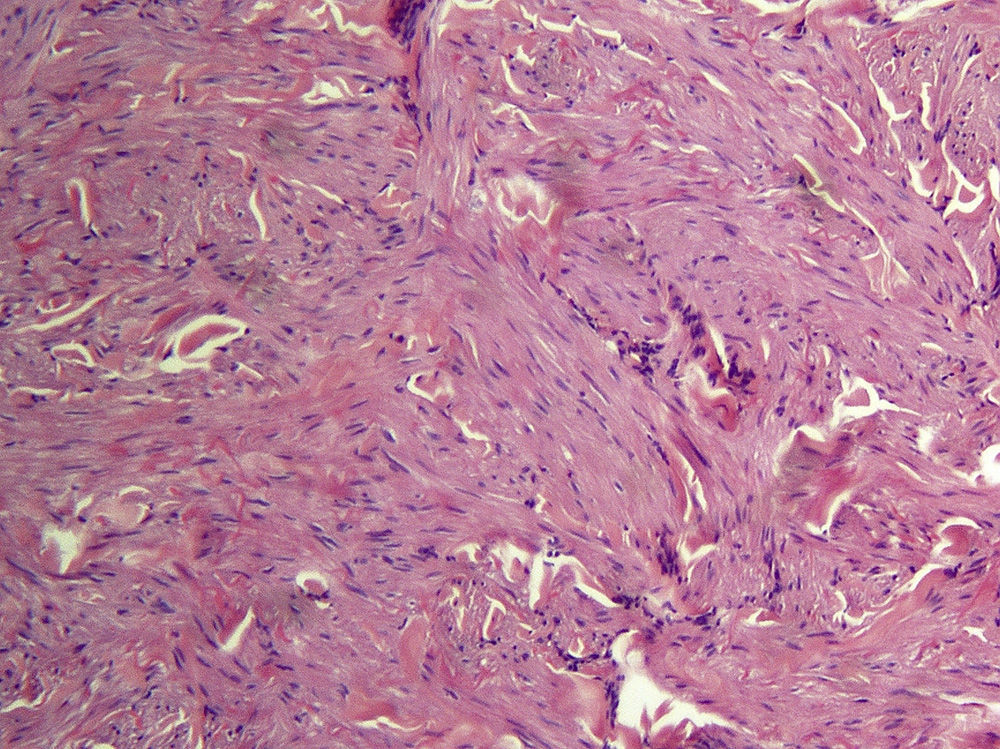
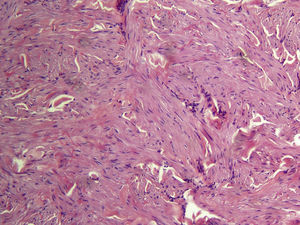
Cutaneous leiomyomas are benign neoplasms that arise from smooth muscle. Depending on where they originate, there are different types of leiomyomas: angioleiomyomas (vascular smooth muscle), genital leiomyomas (smooth muscle in the genital region), and piloleiomyomas (arrector pili muscle). Piloleiomyomas are the most common type of leiomyoma in MCUL,4 and this is supported by our findings. Leiomyomas typically occur as solitary lesions (sporadic), but piloleiomyomas can be solitary or multiple (sporadic or familial). Clinically, cutaneous lesions in MCUL are slow-growing, flesh-colored, brownish, or pink papules or nodules measuring between 0.2 and 2cm. They typically develop between adolescence and the fourth decade of life and can appear before, at the same time, or after uterine fibroids. The trunk and flexor surfaces of the extremities are the most common sites affected and lesions can appear as solitary papules or nodules, isolated lesions in different areas, or groups of lesions in a skin segment.5,6 Coinciding with reports in the literature,7 the most common presentation in our series was type 2 segmental involvement, which is characterized by more severe cutaneous involvement in the segment of skin affected and symmetrically distributed lesions. In our series, there was a slight predominance of patients with asymptomatic lesions, contrasting with reports in the literature describing frequent discomfort or pain,8,9 particularly in response to pressure, changes in temperature, or stress.5 Diagnosis is confirmed histologically, and characteristic findings include smooth muscle bundles interspersed with collagen distributed unevenly through the dermis and sparing the epidermis (Fig. 6). Spindle cells typically have long, cigar-shaped nuclei. Immunohistochemical stains are positive for actin and desmin.10
Most uterine fibroids are sporadic and are the most common tumor in women of reproductive age. Between 76% and 100% of women with MCUL and mutations in the FH gene develop uterine fibroids.8,9,11 These typically appear before the fourth decade of life (mean age, 30 years; range, 18-52 years) and are a common reason for early hysterectomy. (In one series, 44% of the women were aged 30 years or younger.) Our findings support these observations. Progression to a highly aggressive form of leiomyosarcoma has been described,12 but we observed no cases in our series.
There is an increased incidence of renal carcinoma (2%-16% according to the series) in families with MCUL.8,13 Patients with HLRCC have the same mutation as those with MCUL,2,3 but no association has yet been found between a specific mutation and an increased risk of renal carcinoma. The most common type of renal cell carcinoma in HLRCC is type 2 papillary renal cell carcinoma, which is an aggressive tumor with early onset (mean age of diagnosis of 44 years). It is more common in women, tends to be unilateral, and metastasizes in 50% of cases. When it forms part of HLRCC, renal cell carcinoma develops at earlier ages and has a higher rate of metastasis than its sporadic counterpart. Collecting-duct cancer, oncocytoma, clear cell cancer, and Wilms tumor have also been described in association with HLRCC.14 Renal cell carcinoma is diagnosed by imaging studies, preferably computed tomography (CT) or magnetic resonance imaging (MRI), as type 2 papillary renal cell carcinoma tends to be hypoechoic on ultrasound and may be confused with a cyst. We detected no cases of renal cell carcinoma in our series, although it should be noted that screening was not performed by CT or MRI. While it is possible that cases may be missed by ultrasound, none of the patients undergoing ultrasound monitoring in our series have shown changes or developed symptoms. There were 4 cases of benign renal lesions (cysts, calculi, and adrenal nodule) in our series. This is the first time these lesions have been reported in association with MCUL and we are therefore unaware of any possible relationship.
There have been isolated reports of other diseases associated with MCUL, including multiple endocrine neoplasia type 1, rheumatoid arthritis, breast cancer, prostate cancer, bladder cancer, renal and ovarian cysts, and adrenal cortical adenoma.15,16
MCUL is caused by a heterozygous mutation in the FH gene on chromosome 1q42.3-43. This gene codes for FH, a mitochondrial enzyme in the Krebs cycle that catalyzes the conversion of fumarate to malate and could act as a tumor suppressor. MCUL is an autosomal dominant disorder in which most cases are due to a heterozygous germline mutation in the FH gene. Nevertheless, there have been some reports of sporadic or mosaic cases in patients with no evidence of the germline mutation; there was no family history and the patients had segmental cutaneous lesions without uterine fibroids.9,17 There have also been reports of type 2 segmental involvement due to a loss of heterozygosity at the same locus responsible for the more diffuse variant of the disease.7 These patients had diffuse but more pronounced lesions confined to a segment of skin. Homozygous germline mutations (2 copies) result in a completely different phenotype, characterized by neurological dysfunction and relatively short survival (months or a few years).
The largest series to date of MCUL included 46 patients with multiple cutaneous leiomyomas; 89% of these had highly penetrant germline FH mutations.9 The study also investigated relatives of the 46 probands (62 affected and 42 apparently unaffected individuals). Of the 150 people studied, 62% had FH mutations and all the male carriers in this group had cutaneous leiomyomas. In the case of the female carriers, 69% had cutaneous and uterine leiomyomas (fibroids), 15% had cutaneous leiomyomas only, 7% had uterine leiomyomas only, and 9% had no clinical lesions, possibly because of incomplete penetrance.9 In our series, it was not possible to screen for FH mutations.
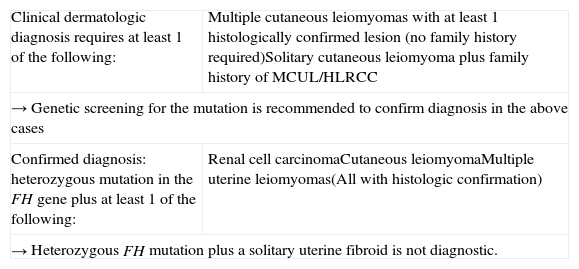
There is still a lack of consensus on the diagnostic criteria for MCUL. In a review published in 2014, Pithukpakorn and Toro18 proposed a series of diagnostic criteria, which are shown in Table 2. Before the existence of the FH mutation was known, multiple cutaneous leiomyomas with at least 1 histologically confirmed lesion, or a solitary cutaneous leiomyoma combined with a family history of MCUL were considered diagnostic for this syndrome. Despite the discovery of the FH mutation, the above diagnostic criteria have not been modified and still hold, but a new criterion has been added, namely, the presence of a heterozygous FH mutation combined with renal cell carcinoma or at least 1 histologically confirmed cutaneous leiomyoma. Given the high prevalence of uterine leiomyomas, however, the presence of a single uterine fibroid is not sufficient for diagnosis, even in the presence of a heterozygous FH mutation.18 Genetic testing for the FH mutation could be an option to confirm a clinical diagnosis. Tests include molecular analysis of the gene or detection of reduced (≤60%) FH enzyme activity in cultured skin fibroblasts or lymphoblasts.18 Genetic counseling should be offered to patients and relatives in the case of a positive diagnosis.
Diagnostic Criteria for MCUL/HLRCC Proposed by Pithkapakorn and Toro in 2014.
| Clinical dermatologic diagnosis requires at least 1 of the following: | Multiple cutaneous leiomyomas with at least 1 histologically confirmed lesion (no family history required)Solitary cutaneous leiomyoma plus family history of MCUL/HLRCC |
| → Genetic screening for the mutation is recommended to confirm diagnosis in the above cases | |
| Confirmed diagnosis: heterozygous mutation in the FH gene plus at least 1 of the following: | Renal cell carcinomaCutaneous leiomyomaMultiple uterine leiomyomas(All with histologic confirmation) |
| → Heterozygous FH mutation plus a solitary uterine fibroid is not diagnostic. | |
Source: Adapted from Pithukpakorn and Toro.18
Abbreviations: FH, fumarate hydratase; HLRCC, hereditary leiomyomatosis; MCUL, multiple cutaneous and uterine leiomyoma.
Screening for noncutaneous manifestations should be considered in first-degree relatives of patients with multiple cutaneous leiomyomas9,18; annual gynecologic examination with ultrasound is recommended for women with multiple cutaneous lesions to facilitate the early detection of subclinical fibroids and aid family planning decisions. There is a lack of consensus on whether screening for renal cell carcinoma is necessary and if so, what technique should be used, as renal ultrasound has low sensitivity for the detection of type II papillary renal cell carcinoma. While some authors have contemplated the option of not screening,9 others recommend more aggressive strategies for patients with a confirmed FH mutation, including CT or MRI studies every year or 2 years from adolescence onwards.18,19
Other authors have proposed a more conservative approach in which yearly or 2-yearly CT or MRI would be indicated only in cases of a family history of renal cell carcinoma.13 While it has been suggested that in the absence of genetic testing patients with MCUL and their relatives should undergo dermatological examination every 1 or 2 years to evaluate the extent of disease and check for lesions suggestive of leiomyosarcoma,18 there have been no reports to date of malignant transformation of cutaneous lesions.
Surgical excision is the treatment of choice for isolated, symptomatic lesions. Electrocoagulation and CO2 laser ablation are also an option, but they are associated with more frequent recurrences. A range of systemic treatments have been proposed to provide pain relief in patients with multiple symptomatic lesions, but they have shown limited effectiveness. Examples are drugs that block smooth muscle contraction (nifedipine,20,21 phenoxybenzamine,22,23 doxazosin,24,25 and nitroglycerin,22), antiepileptics (mirtazapine), opioids (oxycodone), and gabapentin.26 In view of the benign nature of leiomyomas, asymptomatic patients may not need any treatment.
ConclusionsWe have described 13 cases of MCUL with similar clinical and histologic findings to those described in the literature. Type 2 segmental manifestations were the most common presentation. It is important for dermatologists to identify cutaneous leiomyomas and obtain a targeted clinical history to rule out MCUL, as this syndrome is strongly associated with more aggressive uterine fibroids and a risk of renal cell carcinoma. However, consensus is still lacking on the need for screening and on the types of tests required.
Ethical DisclosuresProtection of humans and animalsThe authors declare that no tests were carried out in humans or animals for the purpose of this study.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients and that all patients included in the study have received sufficient information and have given their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Collgros H, Iglesias-Sancho M, Tribó-Boixareu M, Creus-Vila L, Umbert-Millet P, Salleras-Redonnet M. Leiomiomatosis cutánea y uterina múltiple o síndrome de Reed: estudio retrospectivo de 13 casos. Actas Dermosifiliogr. 2015;106:117–125.