Leflunomide is an immunosuppressive agent that has been approved by the United States Food and Drug Administration for the treatment of rheumatoid arthritis and psoriatic arthritis. It is also widely used off-label in other diseases, such as ankylosing spondylitis and systemic lupus erythematosus (SLE). Leflunomide can produce adverse effects, the most common of which are gastrointestinal symptoms, hypertension, and alopecia. Although considered an efficacious and safe drug for the treatment of SLE, leflunomide has been associated with cases of skin rash, such as erythema multiforme1, toxic epidermal necrolysis2, and vasculitis3, and it has even triggered skin lesions in subacute lupus erythematosus4.
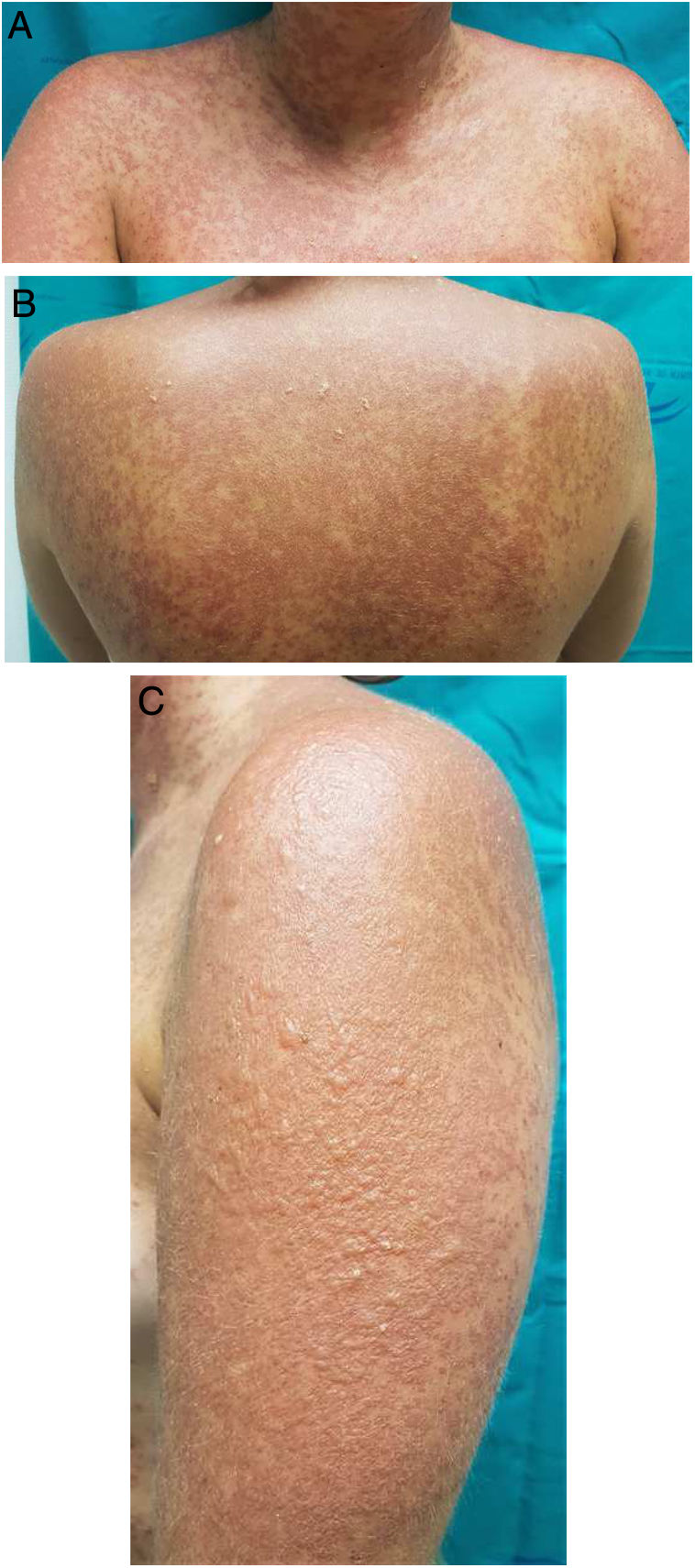
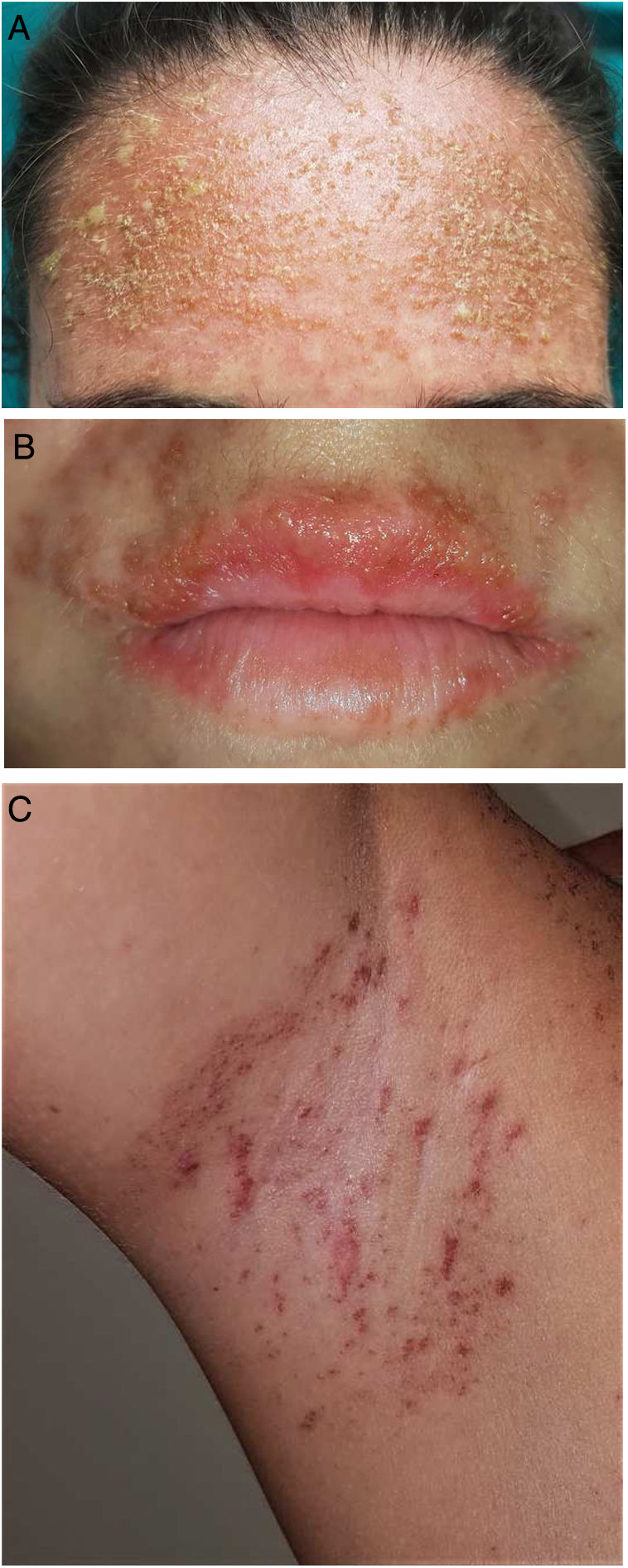
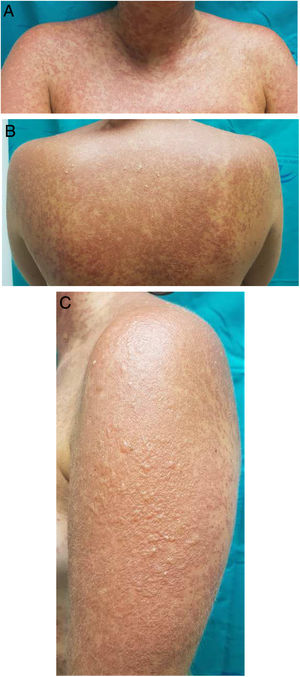
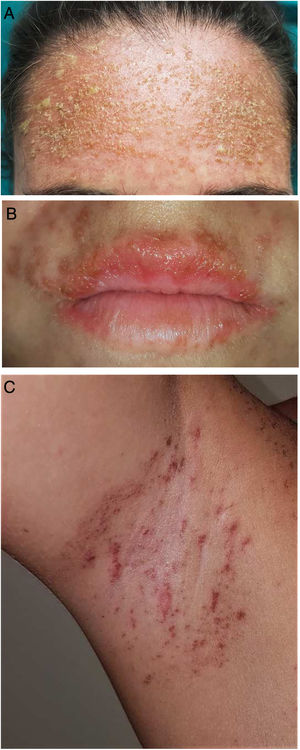
A 25-year-old woman had been followed by the rheumatology department for a 4-year history of SLE. The diagnostic criteria did not include a history of photosensitivity. Initial treatment was with rituximab (500 mg every 15 days, 2 sessions), oral prednisone in tapering doses, and subcutaneous methotrexate (17.5 mg weekly). Owing to gastrointestinal adverse effects, methotrexate was replaced by leflunomide (3 daily doses of 100 mg followed by a maintenance dose of 20 mg/d) 3 months after initiation. Two months after starting treatment with leflunomide and after intense exposure to sunlight at the beach, the patient came to the clinic with a 24 -h history of very pruritic generalized maculopapular rash (Fig. 1A-B), which progressed to vesicular-bullous lesions in 48 hours. These mainly affected the arms (Fig. 1C) and were associated with pustules on the forehead (Fig. 2A), vesicles on the area of the lips (Fig. 2B), and ecchymotic macules and papules on both axillae (Fig. 2C). Examination of the oral and genital mucosa was normal. The laboratory workup revealed high titers for antinuclear antibodies (1/640 U/mL), anti-Ro/SSA-60 (157), anti-Ro/SSA-52 (167), and rheumatoid factor (80 U/mL). Values for the remaining parameters, including C3 and C4, were normal. Serology testing (herpes simplex virus, cytomegalovirus, Epstein-Barr virus, and human herpesvirus 6) yielded negative results. Histopathology of a punch biopsy specimen from a bullous lesion on the posterior trunk revealed an atrophied epidermis with foci of necrotic keratinocytes and spongiosis, together with a subepidermal bullous formation and a moderate inflammatory perivascular mononuclear infiltrate of lymphocytes and eosinophils in the dermis that was compatible with phototoxic rash. Direct immunofluorescence was negative. Treatment with leflunomide was interrupted, and systemic treatment with oral prednisone (1 mg/kg/d) was started. The lesions had resolved completely after 10 days except for some residual hyperpigmentation.
Patients with SLE are particularly sensitive to sunlight, which is considered a trigger or aggravating factor of the disease. It is rare for a diagnosis of SLE not to include photosensitivity as a criterion. Of the various treatments used in SLE, leflunomide is not a first choice; therefore, it is used in selected patients who experience adverse effects associated with other drugs, such as methotrexate. The literature contains many articles that consider leflunomide as the sole cause of subacute cutaneous lupus erythematosus (SCLE)5, as well as SCLE associated with erythema multiforme–type lesions and erythema multiforme major. Some authors are in favor of diagnosing the co-occurrence of lupus erythematosus and erythema multiforme in the same patient as Rowell syndrome, although this is considered controversial. In fact, in the year 2000, Zeitouni et al.6 defined new criteria for the diagnosis of Rowell syndrome. While the patient in the case we report fulfilled sufficient criteria to be diagnosed with this syndrome, our main suspicion was leflunomide-induced phototoxic reaction. Two months after the clinical picture had resolved, the patient underwent patch testing with the European photopatch series (Chemotechnique Diagnostics) and leflunomide 1%, 5%, and 10% in petrolatum, with UV-A radiation at 10 J/cm2. The reading was negative at 3 minutes and at 48 and 96 hours.
Photosensitive rash is common in patients with SLE and is included in the diagnostic criteria. However, in the present case, we think that some of the drugs used in this disease could play a relevant role in triggering a phototoxic or photoallergic reaction. Differentiating clinically between phototoxic rash and photosensitive rash in SLE can prove difficult. Histopathology makes it possible to distinguish patients with true SLE, in which immunofluorescence reveals deposits of immunoglobulin M and C3 at the dermal-epidermal junction, findings that are absent in phototoxic rash. Photopatch testing should be considered as an additional test, with the objective of differentiating between phototoxic and photoallergic reactions. As for laboratory findings, a positive anti-Ro/SSA titer should be considered a risk factor for drug-induced SLE7. Furthermore, antihistone antibodies are detected in more than 95% of cases of drug-induced SLE, although they are also observed in 50%-70% of cases of SLE that are not drug-induced. In the case we report, the histone antibody test was negative, thus pointing us to a phototoxic reaction, as initially suspected.
In conclusion, we think that when assessing a patient with characteristics similar to those described here, the differential diagnosis should include drug-induced photosensitive rash, especially after initiation of treatment such as leflunomide, as in the present case. Additional tests, for example, patch (and photopatch) testing, are essential if we are to make an appropriate diagnosis.
FundingThe authors declare that no funding was received for the present study.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Navarro-Triviño FJ, Lucas-Collado N, Salvatierra-Ossorio J. Erupción fototóxica inducida por leflunomida en una paciente con lupus eritematoso sistémico. Actas Dermosifiliogr. 2021;112:939–941.