Therapeutic options for the treatment of hidradenitis suppurativa (HS) are limited, and are further reduced with increasing disease severity.1,2 Apremilast is a phosphodiesterase 4 inhibitor that exerts an immunomodulatory effect, partially blocking the expression of pro-inflammatory cytokines and inducing expression of anti-inflammatory cytokines.3 It is currently indicated in adult patients diagnosed with plaque psoriasis and/or psoriatic arthritis who do not respond to conventional systemic therapy. Based on the hypothesis that apremilast acts on multiple cells involved in the pathogenesis of HS (T cells, natural killer cells, neutrophils, monocytes, and dendritic cells), several studies have sought to characterize its efficacy in the treatment of this disease.4,5
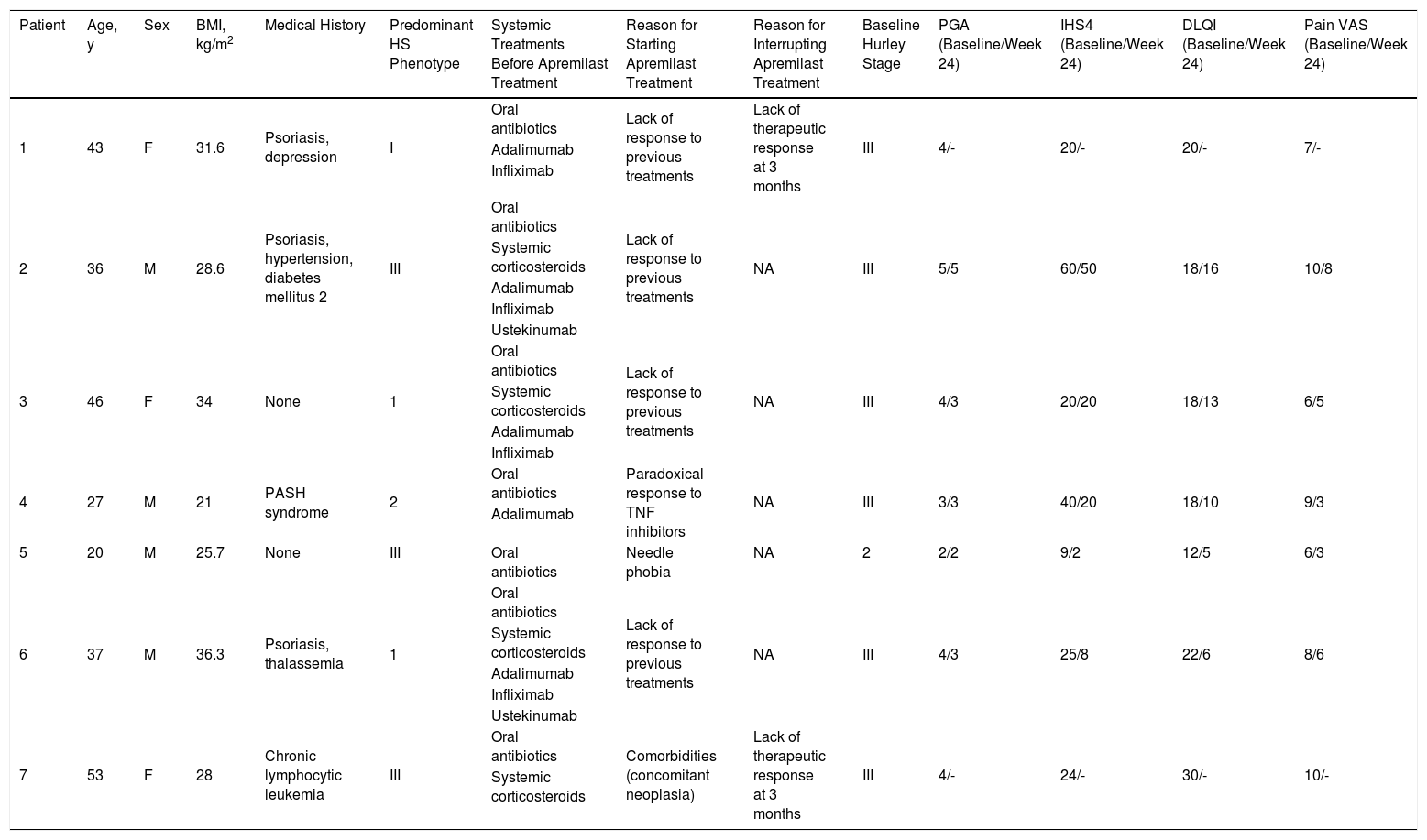
We conducted an observational, descriptive, retrospective study of patients who were diagnosed with HS and treated with apremilast in our hospital between January 2015 and February 2020. Tables 1 and 2 summarize the clinical variables and efficacy and safety findings. Seven patients (3 women and 4 men; mean age, 37.43 y) were recruited. Most had severe disease (Hurley stage III) when treatment began. The predominant phenotypes were type II (axillary) and type III (gluteal). Medical histories of interest included concomitant psoriasis in 3 patients, pyoderma gangrenosum and acne (pyoderma gangrenosum, acne, and suppurative hidradenitis [PASH] syndrome) in 1 patient, and chronic lymphatic leukemia in partial remission in 1 patient. Two patients had received no previous biological treatment, one due to a history of cancer and the other due to needle phobia. Treatment was discontinued in 2 patients at week 12 due to therapeutic failure.
Summary of the Characteristics of the Patients 1–6 Included in our Series (n = 7).
| Patient | Age, y | Sex | BMI, kg/m2 | Medical History | Predominant HS Phenotype | Systemic Treatments Before Apremilast Treatment | Reason for Starting Apremilast Treatment | Reason for Interrupting Apremilast Treatment | Baseline Hurley Stage | PGA (Baseline/Week 24) | IHS4 (Baseline/Week 24) | DLQI (Baseline/Week 24) | Pain VAS (Baseline/Week 24) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 43 | F | 31.6 | Psoriasis, depression | I | Oral antibiotics | Lack of response to previous treatments | Lack of therapeutic response at 3 months | III | 4/- | 20/- | 20/- | 7/- |
| Adalimumab | |||||||||||||
| Infliximab | |||||||||||||
| 2 | 36 | M | 28.6 | Psoriasis, hypertension, diabetes mellitus 2 | III | Oral antibiotics | Lack of response to previous treatments | NA | III | 5/5 | 60/50 | 18/16 | 10/8 |
| Systemic corticosteroids | |||||||||||||
| Adalimumab | |||||||||||||
| Infliximab | |||||||||||||
| Ustekinumab | |||||||||||||
| 3 | 46 | F | 34 | None | 1 | Oral antibiotics | Lack of response to previous treatments | NA | III | 4/3 | 20/20 | 18/13 | 6/5 |
| Systemic corticosteroids | |||||||||||||
| Adalimumab | |||||||||||||
| Infliximab | |||||||||||||
| 4 | 27 | M | 21 | PASH syndrome | 2 | Oral antibiotics | Paradoxical response to TNF inhibitors | NA | III | 3/3 | 40/20 | 18/10 | 9/3 |
| Adalimumab | |||||||||||||
| 5 | 20 | M | 25.7 | None | III | Oral antibiotics | Needle phobia | NA | 2 | 2/2 | 9/2 | 12/5 | 6/3 |
| 6 | 37 | M | 36.3 | Psoriasis, thalassemia | 1 | Oral antibiotics | Lack of response to previous treatments | NA | III | 4/3 | 25/8 | 22/6 | 8/6 |
| Systemic corticosteroids | |||||||||||||
| Adalimumab | |||||||||||||
| Infliximab | |||||||||||||
| Ustekinumab | |||||||||||||
| 7 | 53 | F | 28 | Chronic lymphocytic leukemia | III | Oral antibiotics | Comorbidities (concomitant neoplasia) | Lack of therapeutic response at 3 months | III | 4/- | 24/- | 30/- | 10/- |
| Systemic corticosteroids |
Abbreviations: BMI, body mass index; DLQI, Dermatology Life Quality Index; F, female; IHS4, International HS Severity Score System; M, male; NA, not applicable; PASH, pyoderma gangrenosum, acne, and hidradenitis suppurativa; PGA, Physician’s Global Assessment; TNF, tumor necrosis factor; VAS, pain visual analogical scale.
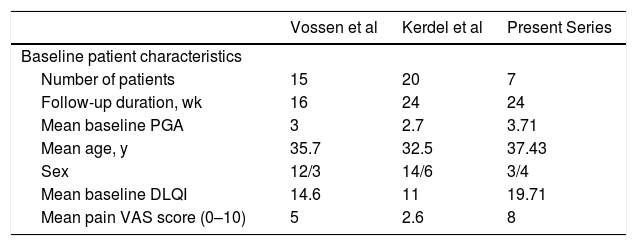
Comparison of the Results of Previous Clinical Trials and of the Present Series.
| Vossen et al | Kerdel et al | Present Series | |
|---|---|---|---|
| Baseline patient characteristics | |||
| Number of patients | 15 | 20 | 7 |
| Follow-up duration, wk | 16 | 24 | 24 |
| Mean baseline PGA | 3 | 2.7 | 3.71 |
| Mean age, y | 35.7 | 32.5 | 37.43 |
| Sex | 12/3 | 14/6 | 3/4 |
| Mean baseline DLQI | 14.6 | 11 | 19.71 |
| Mean pain VAS score (0–10) | 5 | 2.6 | 8 |
| Efficacy and safety | n, % (wk) | n, % (wk) | n, % (wk) |
|---|---|---|---|
| HiSCR50 | 8/15; 53.3 (16) | 12/20; 60 (24) | 3/7; 43 (24) |
| Decrease in pain VAS ≥2 points | – | 6/20; 30 (24) | 4/7; 57 (24) |
| Decrease in PGA ≥1 point | – | 10/20; 50 (24) | 2/7; 29 (24) |
| Decrease in DLQI ≥30% | – | – | 3/7; 43 (24) |
| ≥1 adverse effect | 6/15; 40 (16) | 9/20; 45 (24) | 2/7; 28.6 (24) |
Abbreviations: DLQI, Dermatology Life Quality Index; HiSCR50, hidradenitis suppurativa clinical response; PGA, Physician's Global Assessment; VAS, pain visual analogical scale.
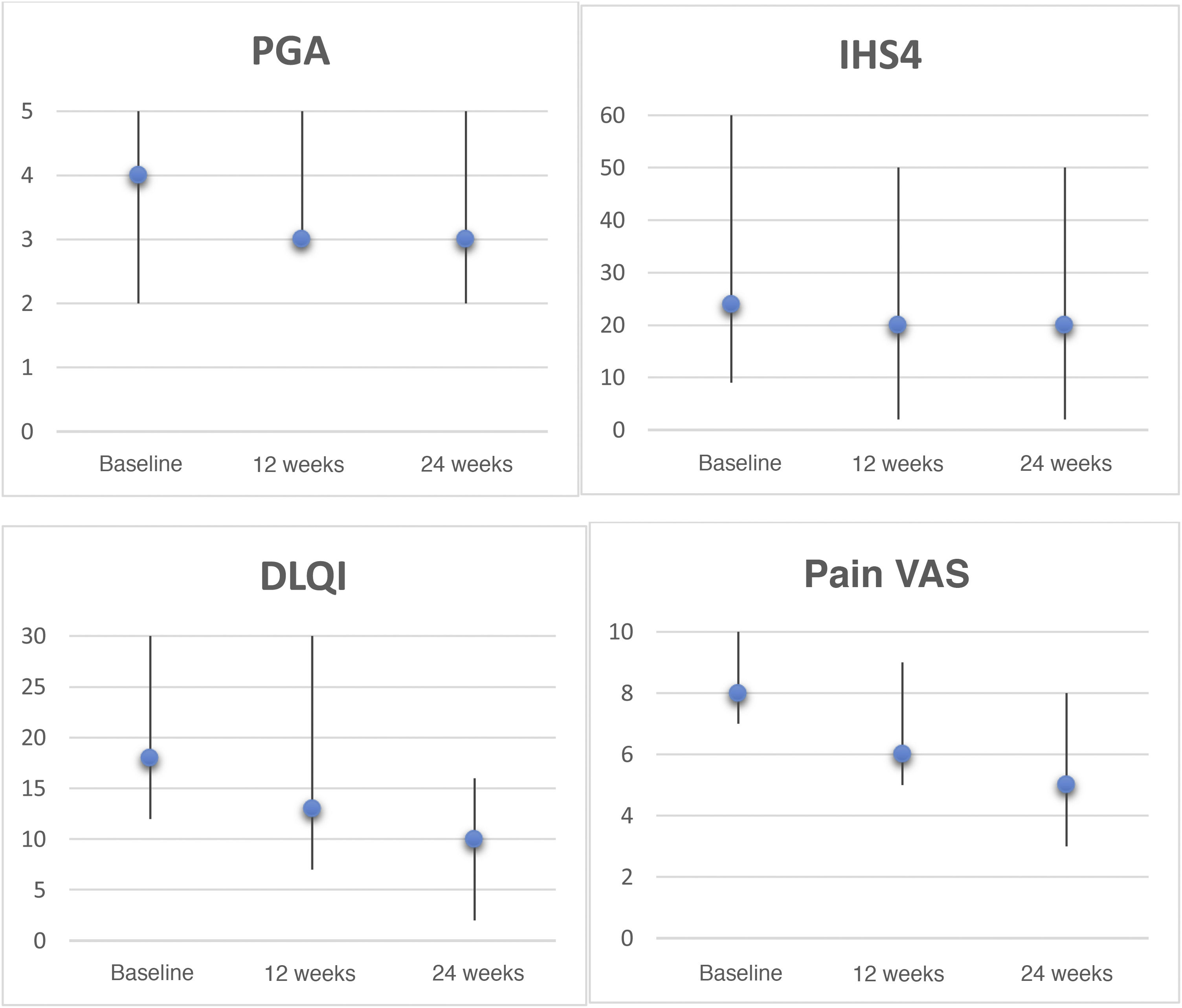
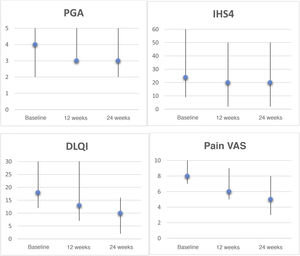
In the intention-to-treat analysis, 43% of patients achieved hidradenitis suppurativa clinical response (HiSCR50) at week 24. We observed a reduction in the pain visual analog scale (VAS) score of ≥2 points in 57% of patients, a reduction in patient global assessment (PGA) score of ≥1 point in 29% of patients, and a decrease in the Dermatology Life Quality Index (DLQI) of ≥30% in 43% of patients at week 24. The evolution of the different response scales (international hidradenitis suppurativa severity score system [IHS4], PGA, pain VAS, and DLQI) at 12 and 24 weeks is shown in Figure 1. Analysis at 6 months revealed a significant reduction in pain (P = 0.042) and the impact of the disease on quality of life (P = 0.043). Only 2 patients had mild gastrointestinal adverse effects that did not require discontinuation of treatment.
Clinical rating scales for hidradenitis suppurativa patients treated with apremilast. Graphs depict the Physician's Global Assessment (PGA), International HS Severity Score System (IHS4), Dermatology Life Quality Index (DLQI), and pain visual analogue scale (VAS, 0–10) scores, expressed as the median and range, at baseline, week 12, and week 24. Statistical analyses were performed to evaluate decreases after 6 months using the Wilcoxon test for paired samples: PGA, P = 0.157; IHS4, P = 0.068; DLQI, P = 0.043; pain VAS, P = 0.042.
In recent years, various published case series have supported the use of apremilast as a therapeutic alternative in patients with HS.3 Moreover, 2 clinical trials have recently been conducted. The first,4 a double-blind randomized clinical trial (n = 15), evaluated the efficacy and tolerance of apremilast for 16 weeks. The second,5 an open phase II clinical trial (n = 20), evaluated efficacy and safety in patients with mild-to-moderate HS for 24 weeks. Both trials reported a similar response rate: 60% of patients achieved HiSCR50 and a reduction of at least 2 points in pain VAS score was observed in 30% of patients by week 24. Up to 45% of the patients experienced at least one adverse effect, the most frequent of which was diarrhea.
Comparison of the baseline characteristics of the patients in our series with those of the aforementioned clinical trials (Table 2) reveals a similar mean age, but a greater proportion of male patients and higher mean baseline PGA and DLQI values in our series. While a similar percentage of patients achieved HiSCR50 across the 3 studies, we observed a considerably greater reduction in pain VAS score at week 24. Therefore, despite the more severe baseline disease and greater impairment of quality of life in our series, the proportion of patients that achieved a therapeutic response was similar to that of the other 2 studies. The retrospective nature of our series may partly explain the lower percentage of adverse effects recorded.
Although psoriasis and HS may share pathogenic mechanisms, the combination of these 2 conditions is rare. We have found only 1 published case describing apremilast treatment of a patient diagnosed with both diseases.6 In our series, 3 patients presented both diseases: a good response of both HS and psoriasis was observed in 2 of these patients, while the third patient stopped treatment due to primary HS treatment failure.
Apremilast could constitute a therapeutic alternative for HS patients who have exhausted other treatment options for which there is greater supporting evidence. We believe that apremilast may be beneficial for the treatment of HS patients with a history of neoplastic disease, paradoxical reactions to tumor necrosis factor (TNF) inhibitors, and active infections, as well as those who decline subcutaneous or parenteral treatments.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Garbayo-Salmons P, Expósito-Serrano V, Ribera Pibernat M, Romaní J. Hidradenitis supurativa tratada con apremilast: serie de casos. Actas Dermosifiliogr. 2021;112:936–939.