Sentinel lymph node (SLN) involvement is the most important prognostic factor in nonmetastatic melanoma. Predictors of nodal involvement include Breslow thickness, ulceration, and mitotic rate.1
The eight edition of the American Joint Committee on Cancer (AJCC-8) melanoma staging system removed mitotic rate as a predictive factor in melanoma because of its poor reproducibility secondary to low intraobserver and interobserver reliability.2 The National Comprehensive Cancer Network Guidelines (version 1.2018), by contrast, suggest that SLN biopsy should be considered in patients with T1a melanoma (<0.8 mm, nonulcerated) and a mitotic rate of > 2 mitoses/mm2, particularly if they are young. There is ample evidence that mitotic rate is predictive of SLN positivity (Table 1). According to a multicenter European study, SLN positivity was the most important prognostic factor in thin melanomas (n = 4249, Breslow thickness < 1 mm), and the only predictor of this positivity was a mitotic rate of > 2 mitoses/mm2. T1a melanoma was associated with an overall risk of SLN positivity of 3.4% (or 1.2% for patients with a mitotic rate of 0 mitoses/mm2), but this risk was 20% for > 2 mitoses/mm2, which is even higher than that observed for T1b melanoma (8%).3 In melanoma patients downstaged to T1a under the AJCC-8 criteria, the 3-year disease-free survival rate was 95%. This rate was significantly lower, however, at 80%, in those with a mitotic rate of > 3 mitoses/mm.4 A US study of 17 204 patients with melanoma with a Breslow thickness of 0.01-1 mm reported a linear relationship between mitotic rate and SLN involvement. After adjustment for known prognostic factors, patients with a rate of > 1 mitoses/mm2 were twice as likely to have SLN involvement than those with < 1 mitoses/mm2. The risk of SLN involvement was 7.9% in patients with 1 mitosis/mm2, but 21.8% and 44.5% for those with 5 and > 10 mitoses/mm2, respectively. A recent European study that included a large cohort for the development (n = 3666) and validation (n = 4227) of a nomogram to improve the selection of patients with thin melanoma (Breslow thickness, < 1 mm) for SLN biopsy showed that age, Breslow thickness, a mitotic rate of > 1 mitosis/mm2, ulceration, lymphovascular invasion, and regression > 75% were all significant predictors of SLN involvement. The resulting nomogram performed better than models based on current international recommendations at identifying which patients with thin melanomas should undergo SLN biopsy and showed that the higher the number of mitoses, the greater the likelihood of SLN involvement.6 Based on these studies, we strongly recommend rigorous assessment of patients with thin melanomas and a high mitotic rate and consider that SLN biopsy should always be contemplated.
Mitotic Rate as a Prognostic Factor in Melanoma.
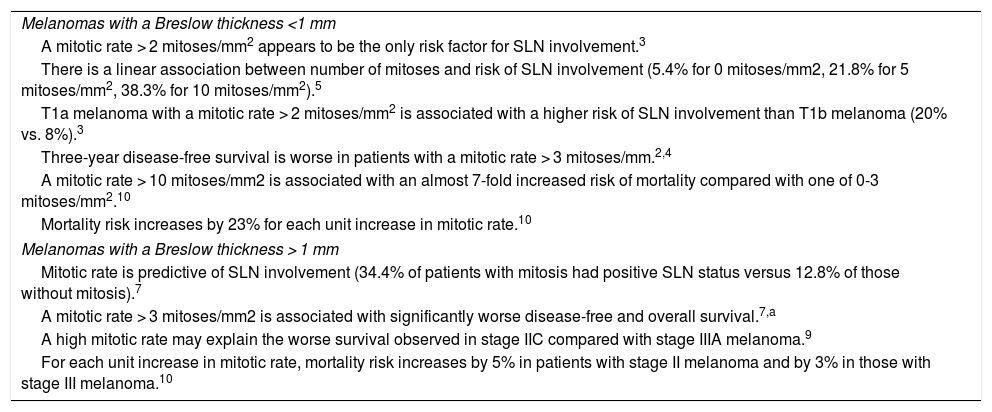
| Melanomas with a Breslow thickness <1 mm |
| A mitotic rate > 2 mitoses/mm2 appears to be the only risk factor for SLN involvement.3 |
| There is a linear association between number of mitoses and risk of SLN involvement (5.4% for 0 mitoses/mm2, 21.8% for 5 mitoses/mm2, 38.3% for 10 mitoses/mm2).5 |
| T1a melanoma with a mitotic rate > 2 mitoses/mm2 is associated with a higher risk of SLN involvement than T1b melanoma (20% vs. 8%).3 |
| Three-year disease-free survival is worse in patients with a mitotic rate > 3 mitoses/mm.2,4 |
| A mitotic rate > 10 mitoses/mm2 is associated with an almost 7-fold increased risk of mortality compared with one of 0-3 mitoses/mm2.10 |
| Mortality risk increases by 23% for each unit increase in mitotic rate.10 |
| Melanomas with a Breslow thickness > 1 mm |
| Mitotic rate is predictive of SLN involvement (34.4% of patients with mitosis had positive SLN status versus 12.8% of those without mitosis).7 |
| A mitotic rate > 3 mitoses/mm2 is associated with significantly worse disease-free and overall survival.7,a |
| A high mitotic rate may explain the worse survival observed in stage IIC compared with stage IIIA melanoma.9 |
| For each unit increase in mitotic rate, mortality risk increases by 5% in patients with stage II melanoma and by 3% in those with stage III melanoma.10 |
Abbreviation: SLN, sentinel lymph node.
The predictive value of mitotic rate is not limited to thin melanomas. One Italian study of 1524 patients with melanoma with a Breslow thickness > 1 mm found a significant association between mitotic rate and SLN involvement. In particular, a rate of > 1 mitosis/mm2 was associated with worse disease-free survival (hazard ratio, 1.82; 95% CI, 1.02-3.24; P = .043).7 Similar results were obtained in a Canadian study of thin melanomas (n = 1072), where a mitotic rate of > 1 mitosis/mm2 was the only associated factor for SLN involvement in patients with a Breslow thickness of 1.01-2.0 mm.1 A Spanish study of 141 patients with a mean Breslow thickness of 2.6 mm reported that ≥ 2 mitoses/mm2 was associated with worse disease-free and overall survival rates.8 Another study of 128 patients attempting to explain the paradoxically worse survival rates observed in patients with stage IIC versus IIIA melanoma found that an age of 55 years or older and a mitotic rate of > 5 mitoses/mm2 were independent predictors of overall survival.9 The authors suggested that stage IIC and IIIA melanomas might be biologically distinct and that mitotic rate should be considered in this subgroup of patients. A large US study of 71 235 patients with melanoma that used 3 cutoff points for mitotic rate (0-3, 4-10 and > 10 mitoses/mm2) found a linear association between mitotic rate and disease-specific survival for stages I, II, and III. In patients with stage I melanoma, the 5-year disease-specific survival rate was 98.3% for patients with a mitotic rate of 0-3 mitoses/mm2 versus 79.7% for those with a rate of > 10 mitoses/mm2. The corresponding rates were 86.1% versus 72.9% for patients with stage II melanoma and 72.5% (0-3 mitoses/mm2) versus 49.7% for those with stage III melanoma. Mortality risk increased by 23% for each unit increase in mitotic rate among patients with stage I disease, and the corresponding increases for patients with stage II and III disease were 5% and 3%, respectively. Patients with stage I disease and > 10 mitoses/mm2 had an almost 7-fold increased risk of mortality compared with those with 0-3 mitoses/mm2.10 A recent Australian study of 156 children and adolescents younger than 20 years with a median Breslow thickness of 1 mm showed that mitotic rate had greater prognostic value than Breslow thickness, and was the only independent predictor of recurrence-free survival.11
Mitotic rate is an important prognostic factor in melanoma. We recommend individualized management of patients with a high mitotic rate, and believe that those with a Breslow thickness of > 1 mm should be considered for SLN biopsy and undergo staging studies and close follow-up. Diagnostic procedures should also be optimized to increase the reproducibility of mitotic rate assessment.
Please cite this article as: Bois MC, Morgado-Carrasco D, Barba PJ, Puig S. El índice mitótico como factor pronóstico y sus implicancias en el manejo del melanoma. Actas Dermosifiliogr. 2021;112:941–943.