Sebaceous adenoma (SA) and sebaceous carcinoma (SC) are uncommon adnexal tumors with sebaceous differentiation. SA is a benign lesion, whereas SC is a malignant neoplasm. Both can appear in isolation or in the context of Muir-Torre syndrome (MTS).1
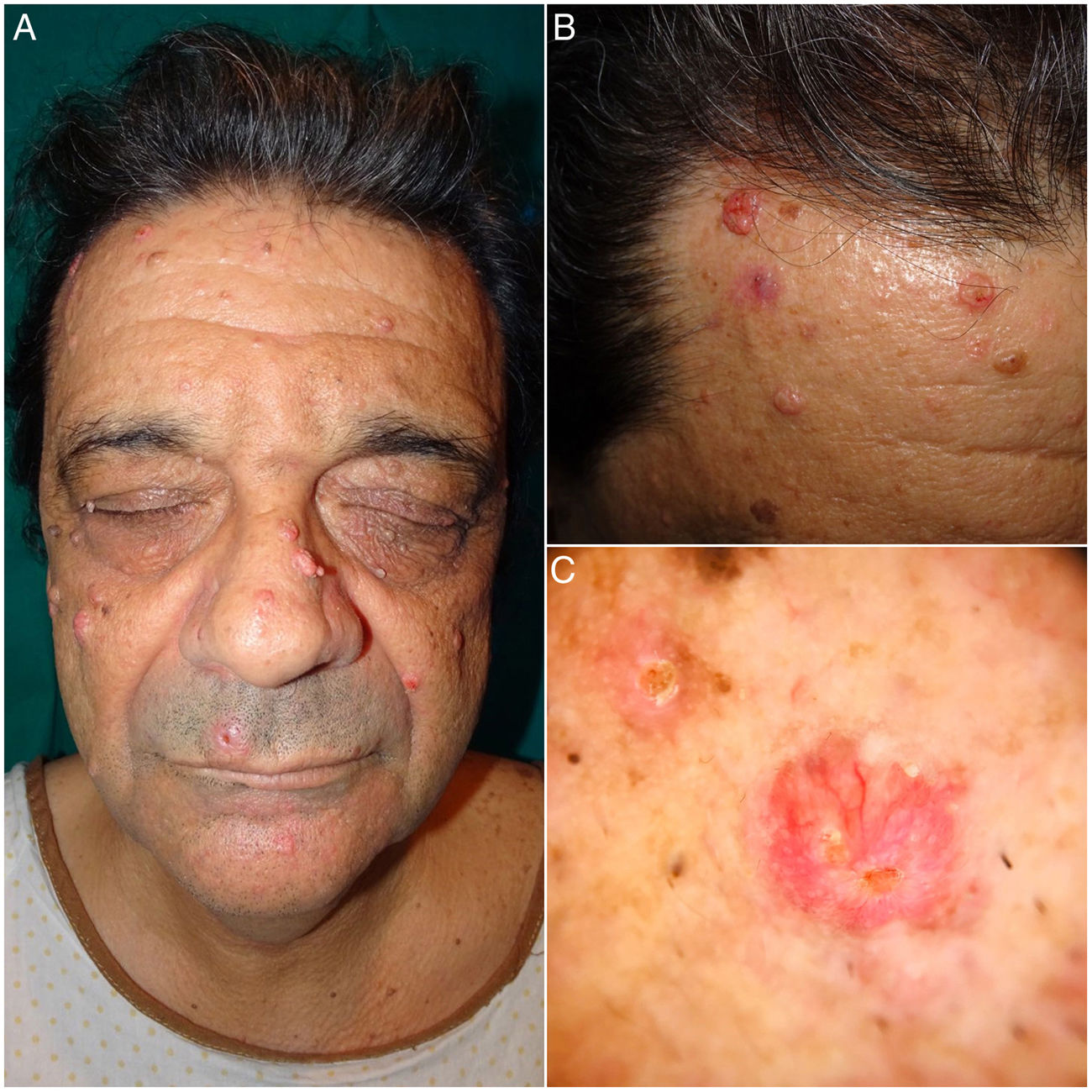
A 60-year-old man consulted for the gradual appearance of asymptomatic facial tumors over the previous 8 years. The patient had received a lung transplant 15 years earlier and was therefore receiving immunosuppressive treatment with tacrolimus and oral corticosteroids, as well as everolimus (last 3 years). The skin lesions stabilized with the introduction of everolimus. The patient had no personal or family history of malignancy of intestinal polyposis.
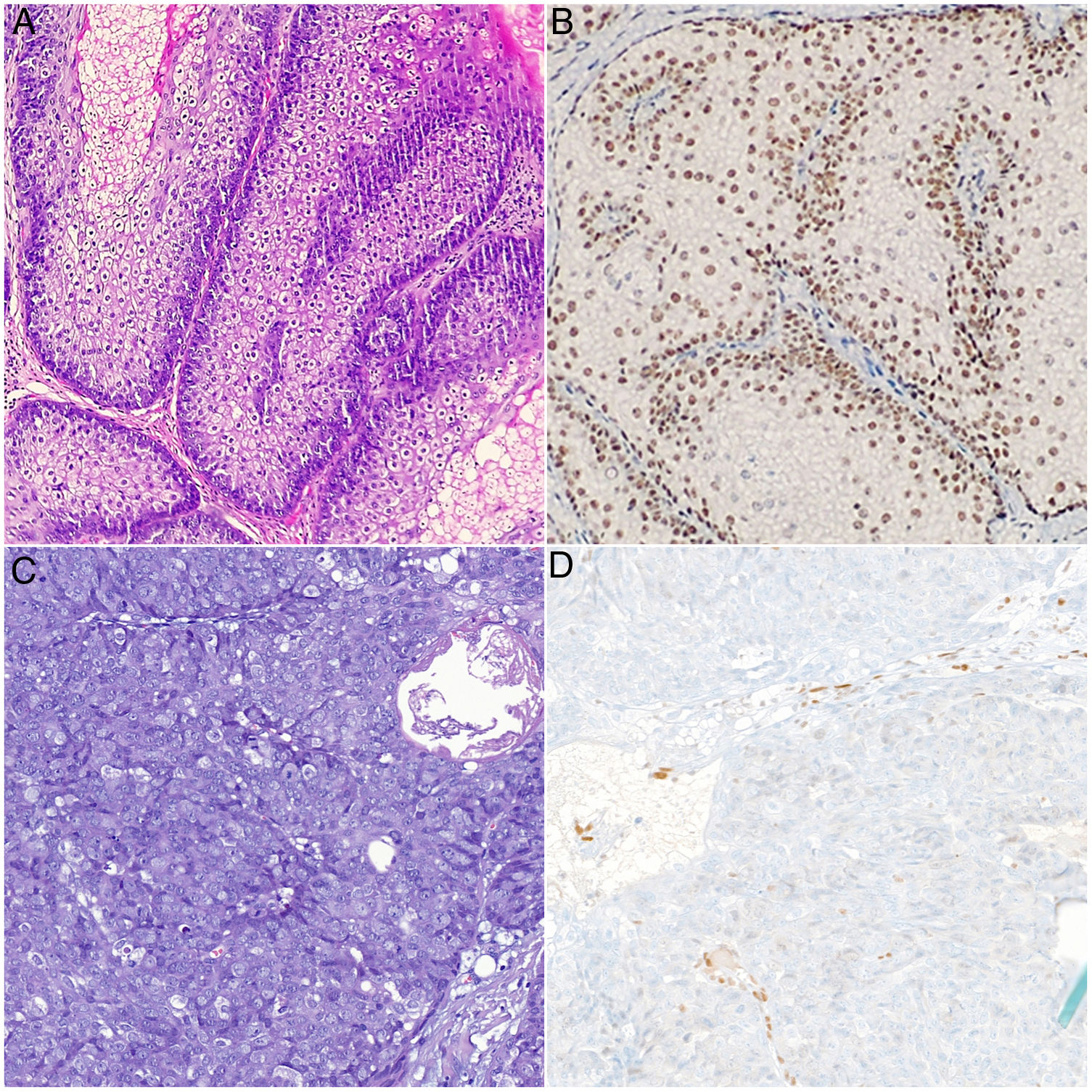
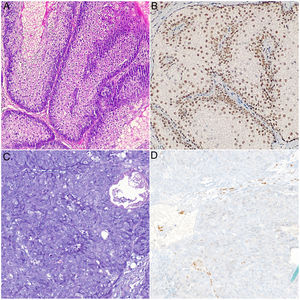
Physical examination revealed multiple umbilicated tumors on the face. These were pinkish in color, measured 5–25mm, and were found mainly on the nasal dorsum, forehead, and cheeks. Dermoscopy revealed the presence of pale yellowish globules on an erythematous background accompanied by branching and tortuous vessels (Fig. 1). Dermatopathology revealed a well-circumscribed tumor formed by sebaceous lobules separated by septa of connective tissue. Each lobule was composed of basaloid cells on the periphery and mature sebaceous cells in the center. Of note was the presence of more disordered maturation than in healthy sebaceous glands, with scant mitotic figures and no necrosis. These findings were consistent with a diagnosis of SA (Fig. 2). Immunohistochemical staining for MLH1, MSH2, MSH6, and PMS2 ruled out microsatellite instability, thus making a diagnosis of associated MTS unlikely (Fig. 2B).
A and B) Histologic images of sebaceous adenoma. Note the sebaceous lobules composed of basaloid cells on the periphery and mature sebaceous cells in the center. These are separated by septa of connective tissue. Intact nuclear positivity for MSH6: A, hematoxylin-eosin, ×20. B, Immunohistochemistry staining for MSH6, ×20. C and D, Histology images of sebaceous carcinoma. Nondifferentiated cells with large nuclei, prominent nucleoli, abundant mitotic figures, and areas of necrosis. Note the loss of nuclear positivity for MSH6 in the tumor cells (C), hematoxylin-eosin, original magnification, ×20; (D) immunohistochemistry staining for MSH6, ×20.
Several lesions were removed at successive check-ups for diagnostic and therapeutic purposes. All of the lesions were reported to be SA, except for a lesion that had first appeared on the area of the maxilla 2 months earlier and was diagnosed as SC. Dermatopathology of this lesion revealed an ulcerated tumor with a lobular pattern composed of nondifferentiated cells with large nuclei, prominent nucleoli, and abundant mitotic figures. The tumor penetrated deeply and showed broad areas of necrosis (Fig. 2C). Immunohistochemistry for detection of DNA replication error repair proteins revealed a loss of nuclear positivity in the tumor cells exclusively for MSH6 (Fig. 2D). Amplification of the margins of the SC was negative, as was cervical and parotid ultrasound, thus making it possible to rule out locoregional involvement. The only finding in colonoscopy for screening of colorectal cancer was an isolated hyperplastic polyp in the area of the rectum. The patient continues to undergo twice-yearly clinical and radiological check-ups.
Solid organ recipients receiving immunosuppressive treatment have a greater risk of developing cutaneous adnexal tumors, including sebaceous tumors. Calcineurin inhibitors such as ciclosporin and tacrolimus lead to their development via interaction with transforming growth factor (TGF) β, overexpression of TGF-β1 and vascular endothelial growth factor C, thus enabling them to interfere in the differentiation and growth of the sebaceous glands.
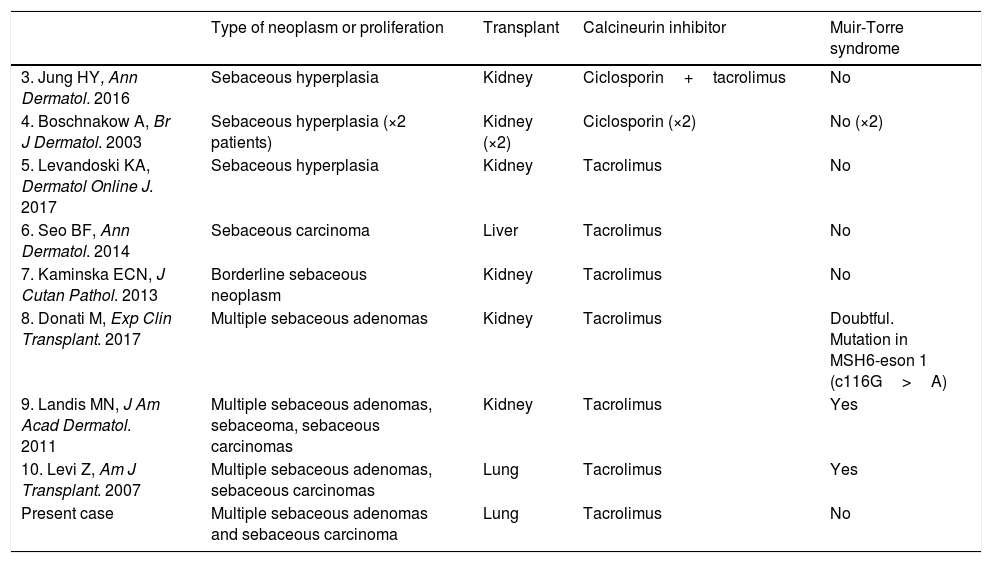
Onset of malignancies and proliferations with sebaceous differentiation in patients taking calcineurin inhibitors is very uncommon. It has been reported sporadically and in association with MTS (Table 1).3–10
Case Reports of Sebaceous Neoplasms in Transplant Recipients Treated With Calcineurin Inhibitors.
| Type of neoplasm or proliferation | Transplant | Calcineurin inhibitor | Muir-Torre syndrome | |
|---|---|---|---|---|
| 3. Jung HY, Ann Dermatol. 2016 | Sebaceous hyperplasia | Kidney | Ciclosporin+tacrolimus | No |
| 4. Boschnakow A, Br J Dermatol. 2003 | Sebaceous hyperplasia (×2 patients) | Kidney (×2) | Ciclosporin (×2) | No (×2) |
| 5. Levandoski KA, Dermatol Online J. 2017 | Sebaceous hyperplasia | Kidney | Tacrolimus | No |
| 6. Seo BF, Ann Dermatol. 2014 | Sebaceous carcinoma | Liver | Tacrolimus | No |
| 7. Kaminska ECN, J Cutan Pathol. 2013 | Borderline sebaceous neoplasm | Kidney | Tacrolimus | No |
| 8. Donati M, Exp Clin Transplant. 2017 | Multiple sebaceous adenomas | Kidney | Tacrolimus | Doubtful. Mutation in MSH6-eson 1 (c116G>A) |
| 9. Landis MN, J Am Acad Dermatol. 2011 | Multiple sebaceous adenomas, sebaceoma, sebaceous carcinomas | Kidney | Tacrolimus | Yes |
| 10. Levi Z, Am J Transplant. 2007 | Multiple sebaceous adenomas, sebaceous carcinomas | Lung | Tacrolimus | Yes |
| Present case | Multiple sebaceous adenomas and sebaceous carcinoma | Lung | Tacrolimus | No |
In the present case, the time sequence suggests that tacrolimus was probably the drug responsible for the pathogenesis of the sebaceous neoplasms. Everolimus does not seem to be associated with the development of these sebaceous neoplasms, given its administration when the lesions had already developed, stabilization of the lesion after initiation, the absence of associated cases in the literature, and its antiproliferative tumor effect via the mTOR pathway. The loss of nuclear positivity for MSH6 in SC is probably a consequence of a sporadic somatic mutation of the malignant cells, given the absence of intestinal polyposis and intact nuclear positivity in immunohistochemistry staining for diagnosis of cutaneous SAs.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Navarro-Navarro I, Jiménez-Gallo D, Tello-Collantes K, Linares-Barrios M. Adenomas sebáceos y carcinoma sebáceo eruptivos inducidos por tacrolimus. Actas Dermosifiliogr. 2021;112:378–381.