Chronic spontaneous urticaria (CSU) has a significant impact on patient quality of life. It is defined as the persistence of wheals, with or without angioedema, for a period of longer than 6 weeks in the absence of an evident trigger. Nonsedating H1 antihistamines are considered the first-line treatment for CSU, but some patients respond poorly, even at doses 4 times the usual dose. Third-line treatments include ciclosporin and omalizumab, a humanized recombinant monoclonal antibody that selectively binds to free immunoglobulin (Ig) E and was recently approved for the treatment of CSU.1–3
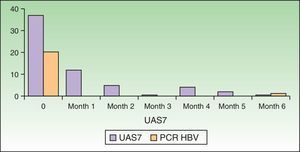
We report the case of a 56-year-old woman with a history of untreated active chronic hepatitis B virus (HBV) infection who had experienced frequent episodes of generalized wheals on a frequent basis over the past 15 years. In the previous year, the wheals had appeared daily. She had been on maintenance treatment with H1 antihistamines (ebastine 20mg/12h) for the past 10 years. In the past year, despite antihistamine therapy, she had required various cycles of oral prednisone to control her symptoms and had visited the emergency department on 2 occasions in the month before being referred to our department. There were no evident triggers. In the initial study, the patient had a viral load of 20200IU/mL and IgE levels of 370kU/L. The other parameters in the workup were within normal ranges. With a diagnosis of CSU, the patient was started on bilastine 40mg/12 combined with a 4-week cycle of prednisone. Response was poor and the symptoms returned on initiation of prednisone tapering. In view of the patient's failure to respond to the first- and second-line treatments and her clinical progress, we decided to step up the intensity of treatment. Following discussion with the hepatology unit, it was agreed that there were no current indications to treat the HBV infection but that it was necessary to avoid immunosuppressants such as ciclosporin. We therefore started treatment with omalizumab 300mg every 4 weeks. The dose of bilastine was gradually reduced. Over the next 6 months, the patient experienced no new episodes of urticaria, even after complete withdrawal of the bilastine. The Urticaria Activity Score 7 (UAS7) decreased from an initial score of 37 (on a scale of 0-42) to a score that consistently ranged between 0 and 5 after the second dose of omalizumab. No adverse effects were observed. There was no evident worsening of the HBV infection and we actually observed a reduction in viral load to 627IU/mL at the end of the 6-month treatment cycle. Fig. 1 shows the changes in UAS7 score and viral loads over the treatment period.
Based on our review of the literature, we believe that this is the first report of the effective and safe use of omalizumab in a patient with CSU and active HBV infection. Brodska and Schmid-Grendelmeier4 described the case of a patient with cold urticaria and chronic HBV infection treated with omalizumab. The treatment was effective and well tolerated, but no information was provided on the clinical features of the infection or on subsequent progress. Antonicelli et al.5 described the case of a patient with HCV infection who achieved asthma control after 19 months of treatment with omalizumab. There was no worsening of liver function and the patient subsequently underwent HVC eradication treatment with interferon and ribavarin. Finally, Leiva-Salinas et al.6 published a case in which omalizumab proved to be both effective and safe in a patient with CSU and HVC infection. No worsening of liver disease was observed, but the authors did not provide information on viral loads. Omalizumab was effective in our patient and did not worsen the underlying HBV infection. It was, in fact, even associated with a reduction in viral load. We cannot provide a clear explanation for this reduction, but other authors have described improved interferon-α immune response to rhinovirus in patients on interferon-α therapy.7
Omalizumab may be an effective and safe treatment alternative for refractory CSU in patients with HVB infection. There is, however, no evidence as yet on possible adverse effects or worsening of viral infection in our patient or in similar cases.
Conflicts of InterestP. Chicharro and P. Rodríguez-Jiménez declare that they have no conflicts of interest. D. de Argila has worked as a clinical advisor and participated in clinical trials sponsored by Novartis.
Please cite this article as: Chicharro P, Rodríguez-Jiménez P, de Argila D. Eficacia y seguridad de omalizumab en un paciente con urticaria crónica espontánea e infección activa por el virus de la hepatitis B. Actas Dermosifiliogr. 2017;108:383–384.