The aim of this review is to familiarize dermatologists and clinicians in general with cutaneous signs and symptoms that can help lead to an early diagnosis of an underlying malignancy. Because the skin is one of the most accessible organs, it should never be overlooked in systemic disease. Examination of the skin has the advantage of revealing important information about the patient's condition without requiring the use of invasive techniques.
In the literature, most discussions of cutaneous manifestations of internal malignancy refer to classic paraneoplastic syndromes, but a wide variety of skin conditions, while not strictly paraneoplastic, can, in certain contexts, indicate the presence of malignancy or an increased risk of developing cancer later in life.
In this review, various skin conditions that can signal malignancy or increased cancer risk are presented in randomly ordered groups based on clinical morphology.
Conditions with multiple signs and symptoms have been classified on the basis of their most characteristic feature.
El objetivo de esta revisión es alertar al dermatólogo y a los clínicos en general de los signos y síntomas cutáneos que pueden contribuir al diagnóstico precoz de una neoplasia subyacente. Puesto que la piel es uno de los órganos más accesible, nunca debe ser ignorada en las enfermedades sistémicas. Posee la ventaja de que su exploración no requiere técnicas agresivas y nos revela datos importantes de la situación del paciente.
Habitualmente se abordan los síndromes paraneoplásicos clásicos, pero hay una gran variedad de procesos cutáneos no estrictamente paraneoplásicos, que en un determinado contexto sugieren la presencia de una malignidad o bien tienen más riesgo de desarrollarla a lo largo de la vida.
Se ha optado por enumerar las distintas dermatosis agrupándolas por su morfología clínica, siguiendo un orden aleatorio.
Varios de los procesos comparten signos y síntomas múltiples por lo que se ha recurrido al más notorio para incluirlo en un determinado grupo.
The skin should never be overlooked in systemic diseases, as it is perhaps the most accessible organ in the body and can be examined using noninvasive techniques.
Furthermore, it can provide the first clues to a diagnosis in 1% of internal malignancies. These clues, combined with a thorough clinical history, can play an important role in alerting the clinician to the presence of an underlying tumor.
Cutaneous manifestations of internal malignancies may be due to direct effects, i.e., the invasion of the skin by a tumor or its metastases, or to indirect effects that trigger the cutaneous signs or symptoms but are not a direct manifestation of the primary tumor. While some of these manifestations occur in isolation, others form part of complex paraneoplastic syndromes. In both cases, however, they can indicate the presence of an underlying malignancy.
In 1976, Curth proposed a set of criteria for establishing a link between skin conditions and internal malignancies. The current definition of a paraneoplastic syndrome is a condition that precedes (the tumor may be present but not yet diagnosed), occurs with, or develops after a malignancy in the absence of evident tumor cells; the conditions run a parallel course. The syndrome itself is not cancerous. Paraneoplastic syndromes are estimated to occur in 7% to 15% of malignancies. Cutaneous manifestations are a reflection of the interaction between tumor cells (from the primary tumor or its metastases) and the host. The pathogenesis of these syndromes is not yet fully understood, but they are believed to be caused by the excessive production or depletion of bioactive substances, growth factors, hormones, or other unidentified mediators produced by the tumor or by antigen-antibody interactions with an aberrant host response to various types of cancer.
It is often difficult to establish a causal relationship between the tumor and the paraneoplastic syndrome, because the temporal relationship is variable. Paraneoplastic syndromes affecting the skin may precede the appearance of the tumor, but they may also manifest much later. This is because in some cases, for symptoms to occur, the tumor must reach a certain mass or level of cell differentiation.1–7
In this review, we analyze cutaneous signs and symptoms that can lead to a diagnosis of an underlying malignancy, regardless of whether or not they form part of a paraneoplastic syndrome. We look at a wide range of conditions, some of which are considered classic paraneoplastic syndromes (marked with an asterisk [*]) while others correspond to a diverse group of skin conditions frequently seen in routine practice. While these latter conditions do not normally indicate malignancy, in certain situations and with certain features, they can alert the dermatologist to the presence of an underlying tumor. In all cases, a high index of clinical suspicion is required. In suspected cases, the clinician will be responsible for ordering additional tests, if warranted, and for monitoring the patient. This is particularly important in conditions that are not commonly associated with malignancy or that are associated with a higher risk of certain tumors. We have used 2 asterisks (**) to denote conditions that are occasionally associated with an internal malignancy and 3 asterisks (***) to denote those that are only rarely associated (Table 1).
We have chosen to group the signs and symptoms by clinical morphology. Given the difficulty of deciding on an order for this list, we opted to list the groups randomly (Table 2). Furthermore, some complex entities have various manifestations that belong to different groups. In such cases, the classification is based on the best known sign or symptom.
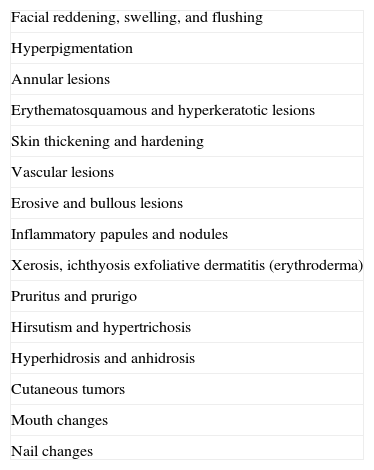
Possible Cutaneous Signs of an Internal Malignancy.
| Facial reddening, swelling, and flushing |
| Hyperpigmentation |
| Annular lesions |
| Erythematosquamous and hyperkeratotic lesions |
| Skin thickening and hardening |
| Vascular lesions |
| Erosive and bullous lesions |
| Inflammatory papules and nodules |
| Xerosis, ichthyosis exfoliative dermatitis (erythroderma) |
| Pruritus and prurigo |
| Hirsutism and hypertrichosis |
| Hyperhidrosis and anhidrosis |
| Cutaneous tumors |
| Mouth changes |
| Nail changes |
This review has been divided into 2 parts, the first of which will look at the first 6 groups shown in Table 2.
Facial Reddening, Swelling, and FlushingEpisodic reddening of the face, known as flushing, is a relatively common symptom seen in many skin disorders. Some of these disorders are harmless, but flushing can be a key indicator of a malignant condition. The most common conditions are detailed below and summarized in Table 3.
Conditions Involving Facial Reddening, Swelling or Flushing Potentially Associated With an Internal Malignancy.
| Carcinoid syndrome** |
| Pheochromocytoma** |
| Superior vena cava syndrome** |
| Mastocytosis** |
| Dermatomyositis** |
| Systemic lupus erythematosus*** |
| Diverse tumors*** |
| Bloom syndrome, Rothmund-Thompson syndrome*** |
** Occasionally associated with an internal malignancy. *** Exceptionally associated with an internal malignancy.
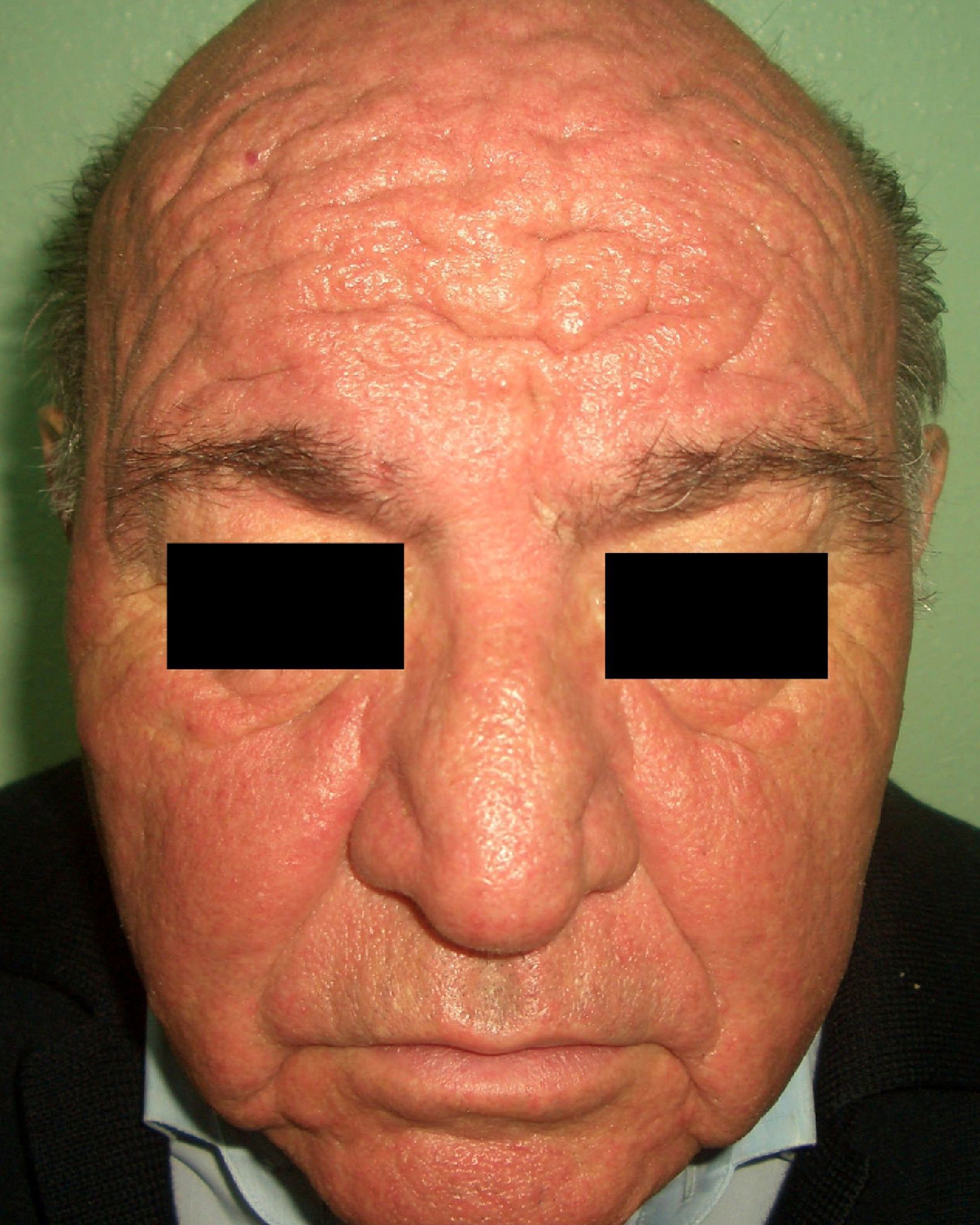
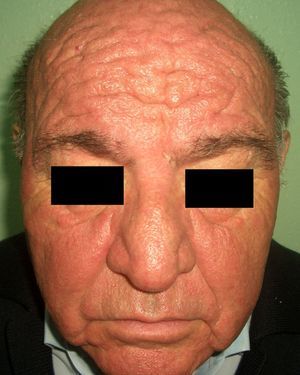
Patients with carcinoid syndrome (**) have a neuroendocrine tumor that secretes vasoactive substances, such as serotonin, histamine, bradykinin, and prostaglandins, that are responsible for the clinical manifestations of this syndrome. Its presence almost always indicates liver metastasis. It is characterized by repeated episodes of flushing, which can progress to severe rosacea, and in some cases even to leonine facies due to metophyma, rhinophyma, and zygophyma (Fig. 1). In more advanced cases, patients may also develop hyperpigmentation, pellagra-like lesions due to tryptophan depletion (hyperkeratosis, xerosis, scaling, glossitis), pachydermoperiostosis (with thickening of the skin) and digital clubbing, scleroderma-like lesions, brown-orange plaques, pruritus, erythema annulare centrifugum, acrocyanosis, and pyoderma gangrenosum. Systemic manifestations include diarrhea, weight loss, cough, dyspnea, and bronchospasms. Diagnosis is confirmed by elevated levels of 5-hydroxyindoleacetic acid in a 24-hour urine test.7–9
Pheochromocytoma (**) is a catecholamine-secreting neuroendocrine tumor derived from chromaffin cells. In 80% of cases, it originates in the adrenal marrow, but ectopic tumors can also occur.
Flushing appears after episodes of hypertension, tachycardia, palpitations, chest pain, throbbing headache, excessive sweating, and facial pallor. The detection of vanillylmandelic acid and catecholamine, usually in high levels, in urine, confirms the diagnosis. Pheochromocytoma is more common in patients with neurofibromatosis type 1, which is not always malignant. Another cutaneous feature is Addison-like hyperpigmentation, which is caused by the ectopic secretion of adrenocorticotropic hormone (ACTH) by the tumor and the production of melanocyte stimulating hormone (MSH); the pigmentation fades after surgical removal of the tumor.10
Facial swelling can alert the dermatologist to a possible diagnosis of superior vena cava syndrome (**). This syndrome can be caused by extrinsic compression of the superior vena cava by a tumor or by intrinsic thrombosis due to a blood clot.
It is characterized by a triad of signs: a) swelling of the face, neck, upper trunk, and arms; b) facial cyanosis, due to venous stasis; and c) collateral circulation in the thoracic and brachial areas. If the syndrome progresses, patients can develop jugular distension, dyspnea, orthopnea, cough, chest pain, and symptoms of cerebral edema (dizziness, nausea, and blurred vision).
Superior vena cava syndrome is caused by a tumor in 85% to 90% of cases. The leading cause is lung cancer, particularly small-cell lung cancer in the right lung. The next most common causes are non-Hodgkin lymphoma, germ cell tumors, thymomas, and metastatic tumors (from breast cancer in most cases).11,12
Flushing can also be a manifestation of mastocytosis (**). Aggressive or systemic forms of this disease can sometimes progress to mast cell leukemia. Flushing in mastocytosis is attributed to the degranulation of mast cells containing substances such as histamine, heparin, cytokines, and tumor necrosis factor. Clinical suspicion is based on the patient's history, and diagnosis can be confirmed by biopsy of skin lesions, if present. Serum tryptase levels should also be determined and a bone marrow biopsy performed only in patients with signs of a hematologic disorder or aggressive mast cells, which are commonly associated with flushing.7,13
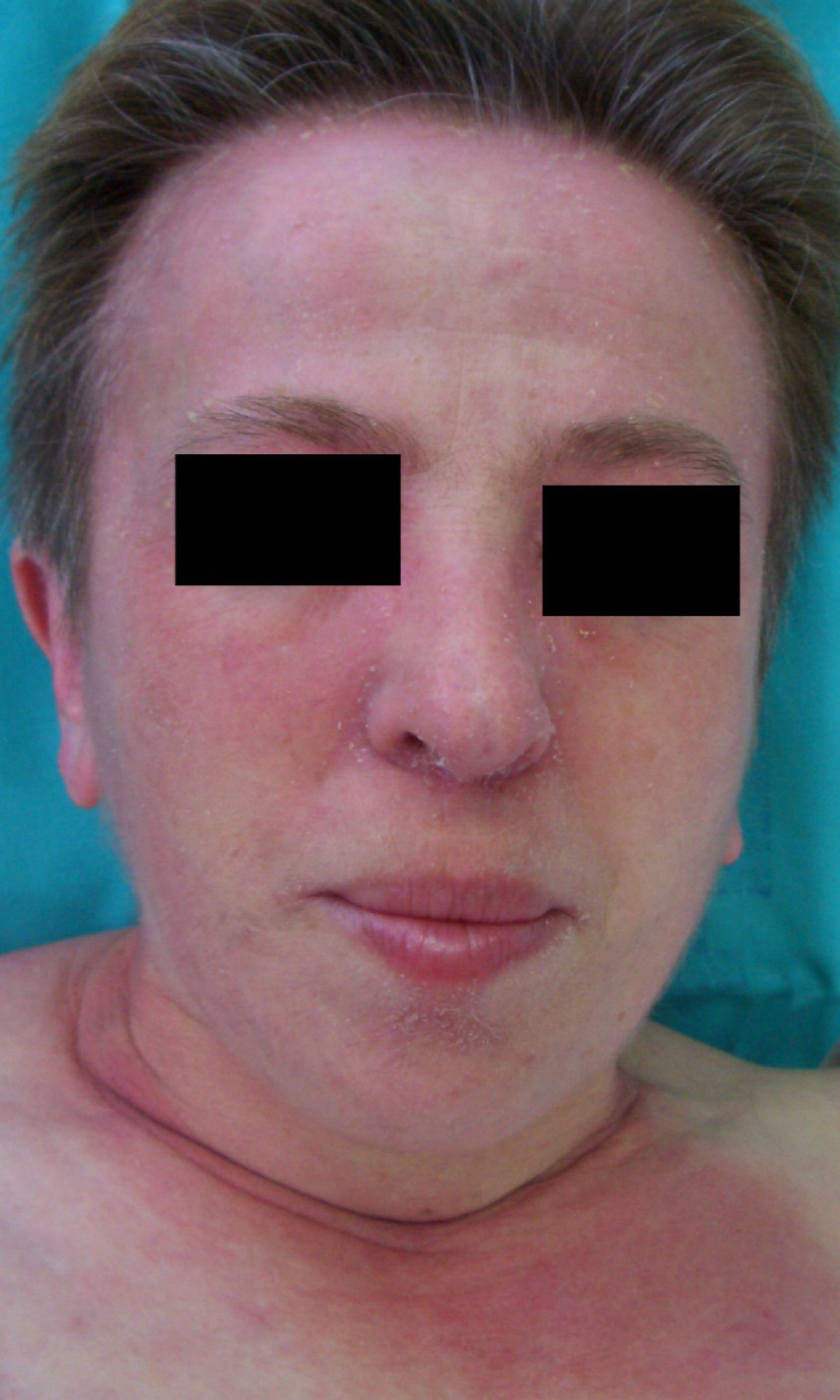
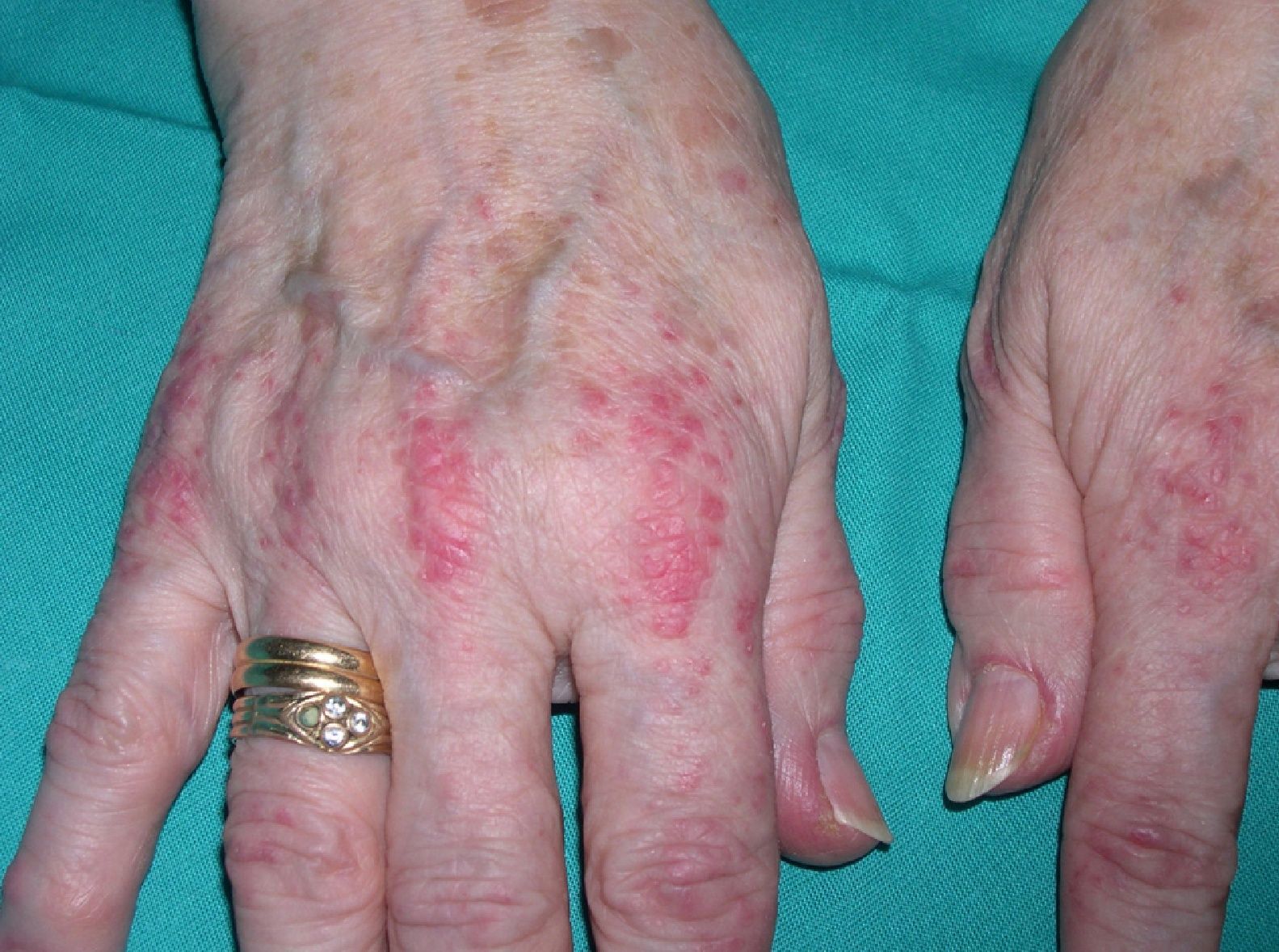
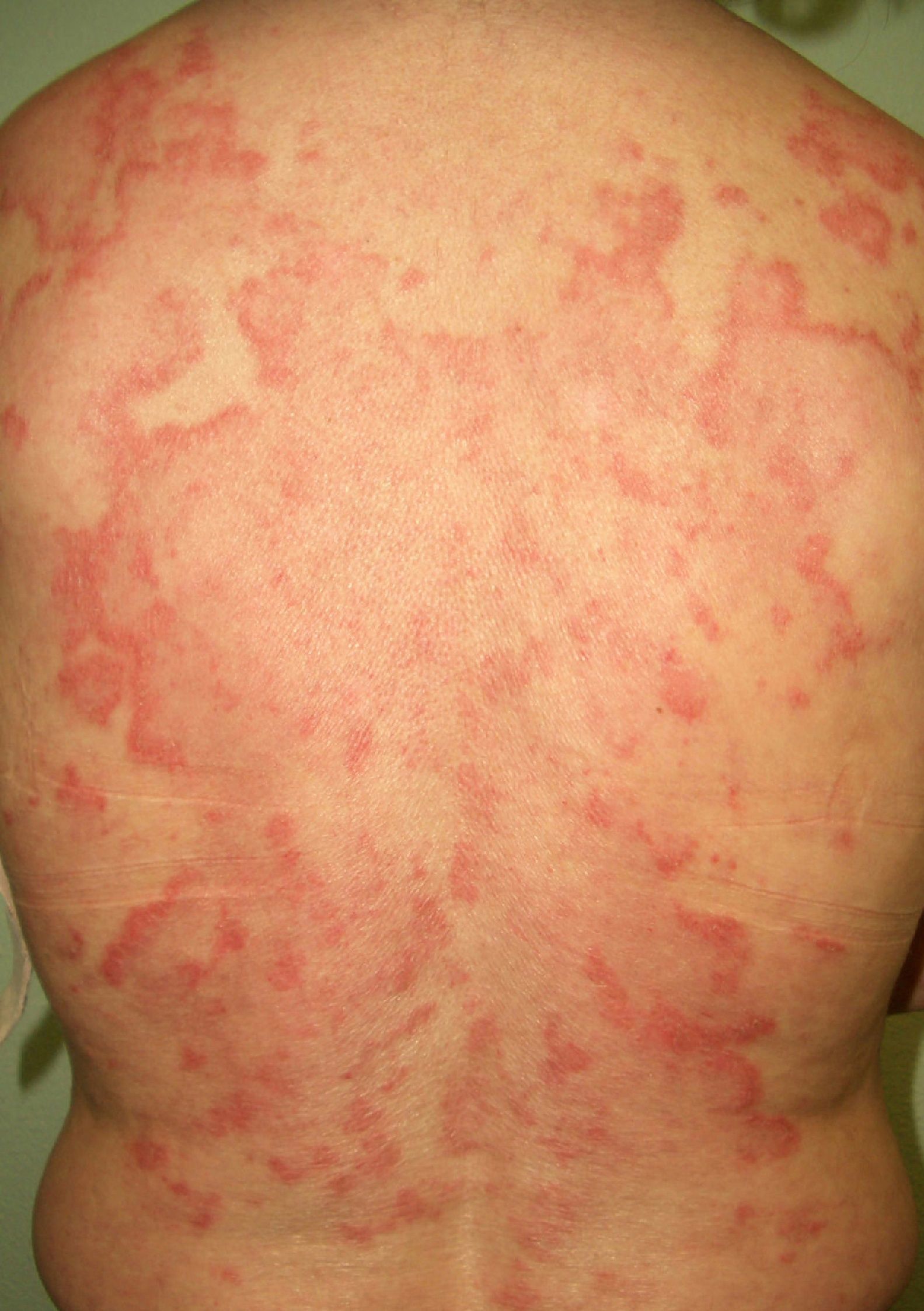
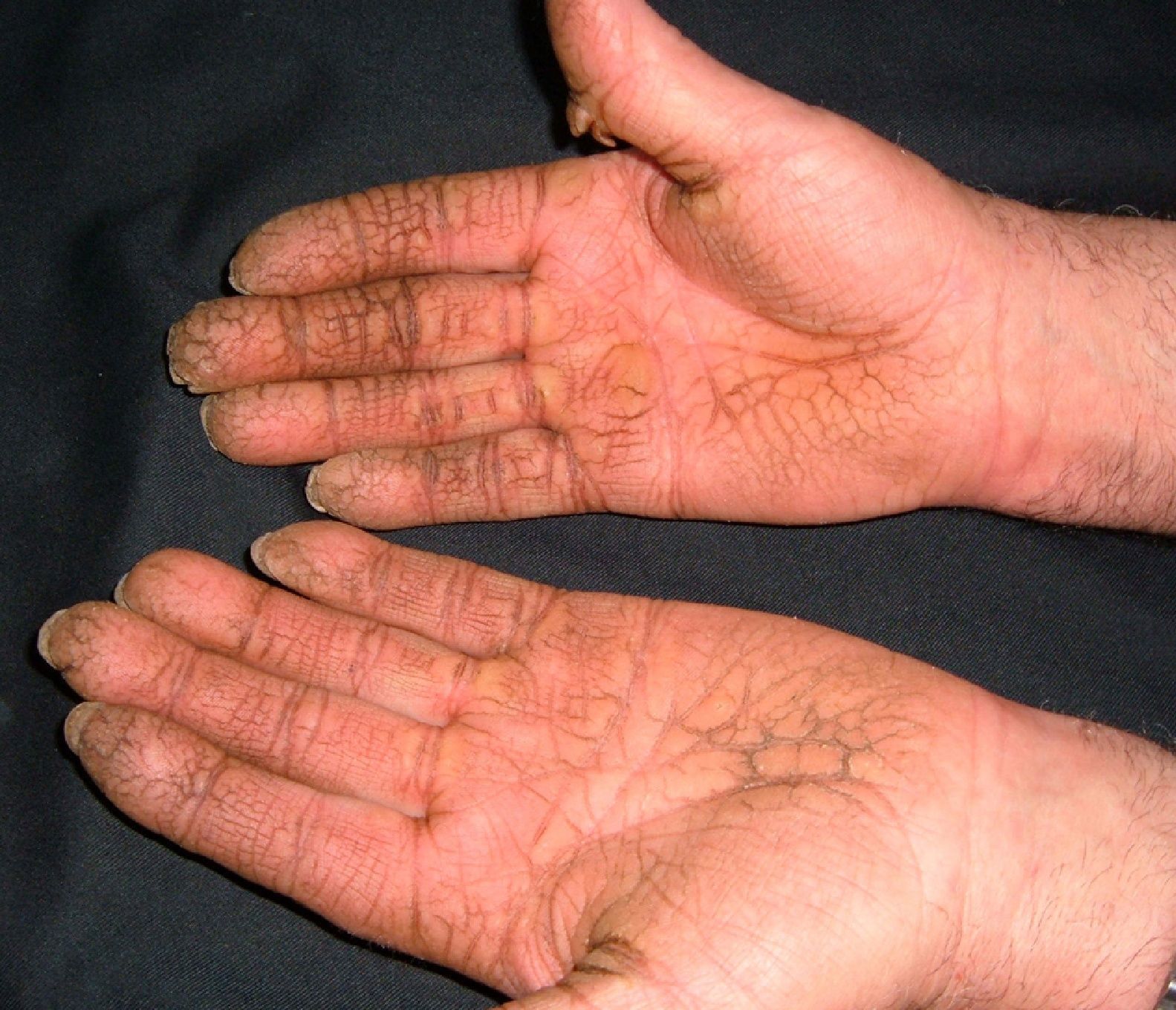
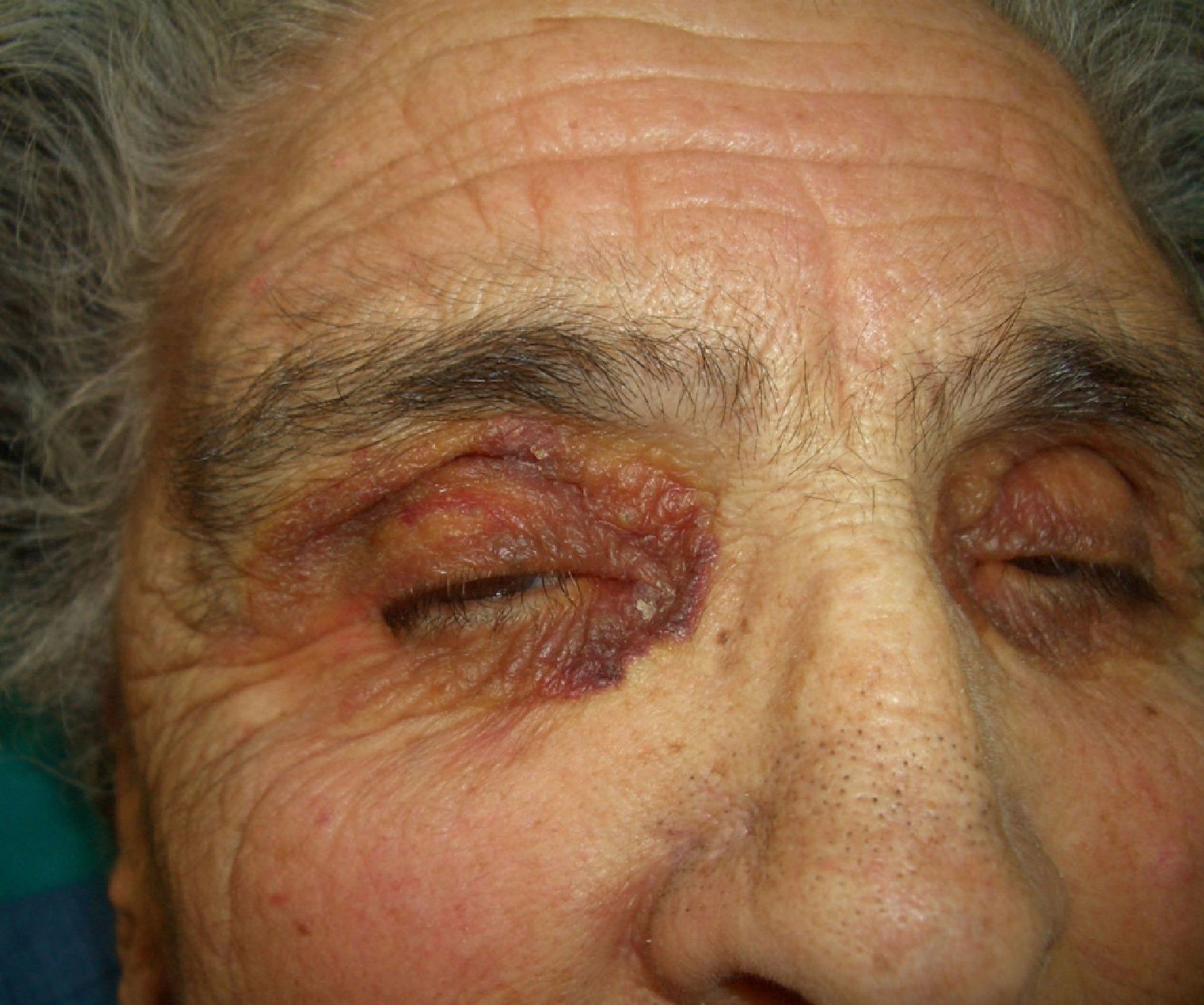
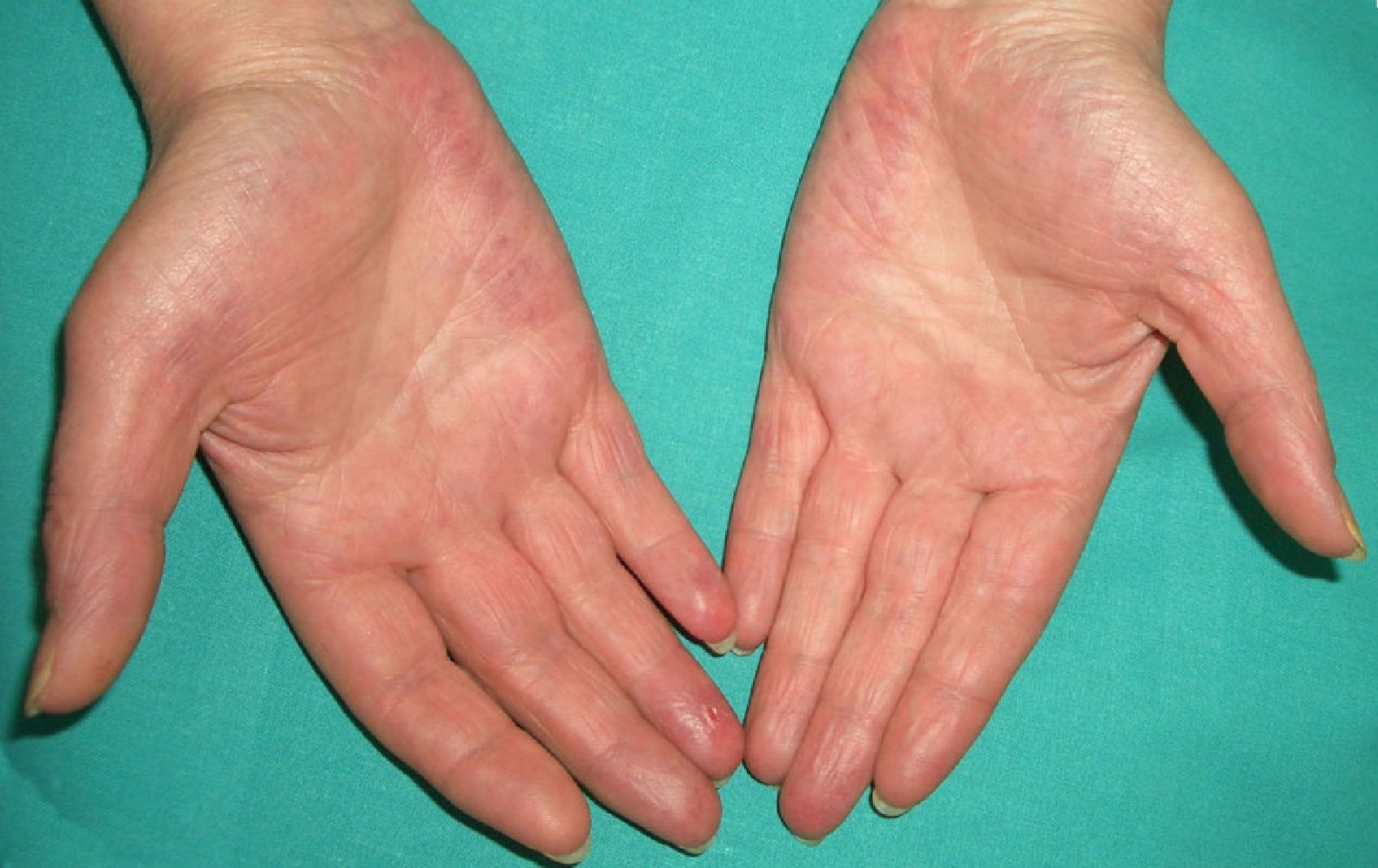
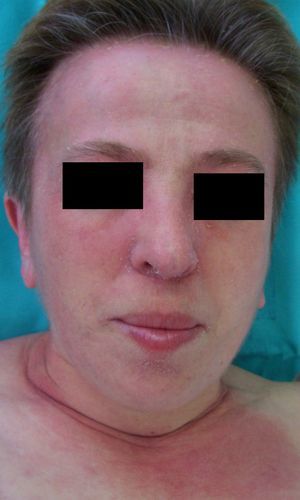
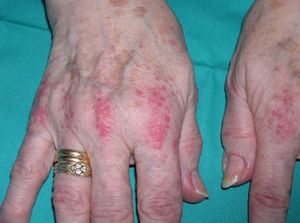
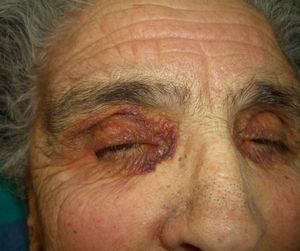
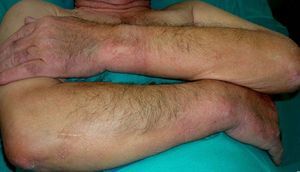
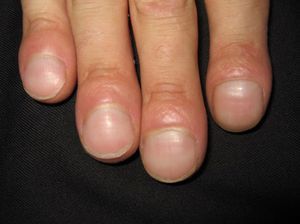
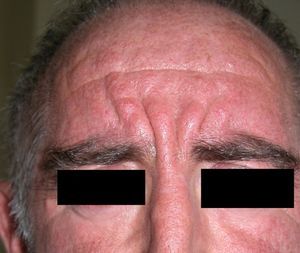
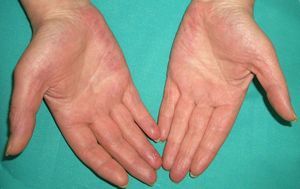
Persistent rather than episodic facial reddening and swelling are occasionally an early sign of dermatomyositis (**), which manifests with swollen, heliotrope eyelids, malar erythema, and photosensitivity (Fig. 2), in addition to characteristic lesions on the hands (Fig. 3), poikiloderma, and nail-fold telangiectasias. The development of vesiculobullous lesions14 and associated cutaneous necrosis15,16 is closely associated with malignancy. The disease can occur with or without myositis, which causes weakness of the proximal muscles.
Approximately 10% to 30% of adult patients with dermatomyositis have an associated malignancy, which can appear before, during, or after diagnosis of the muscle disease. The most common malignancies are ovarian and breast cancer in women17 and lung and prostate cancer in men, although gastrointestinal and ureteral tumors,18 and nasopharyngeal carcinomas19 are also seen. Raynaud phenomenon is not common in paraneoplastic dermatomyositis. Because response to treatment with corticosteroids is poor, immunosuppressive therapy is necessary. Juvenile dermatomyositis is rarely associated with an underlying malignancy.20,21
Adults diagnosed with dermatomyositis should undergo screening for an occult malignancy at least once a year for 3 years.2,3,17
Continuing in the area of connective tissue diseases, systemic lupus erythematosus (SLE) lesions (**), together with facial reddening and the characteristic systemic manifestations and laboratory abnormalities seen in SLE, have been described in patients with intravascular lymphoma and Hodgkin and non-Hodgkin lymphoma.22
Less commonly, facial reddening and swelling (***) can be a sign of a brain tumor, medullary thyroid cancer (secretion of bioactive substances in association with diarrhea and bone metastasis), renal carcinoma (secretion of hormonal substances that cause flushing), or a pancreatic tumor (secretion of various substances that also cause diarrhea).7,13
Facial reddening can also be an important feature of certain hereditary immunodeficiency disorders. Of note in this category are Bloom syndrome and Rothmund-Thomson syndrome (***), which both manifest with reddening of the face and photosensitivity. Patients with Bloom syndrome have an increased risk of leukemia, lymphoma, and gastrointestinal adenocarcinoma. Other cutaneous manifestations seen in Rothmund-Thomson syndrome are telangiectasia and atrophic skin on the face (poikiloderma appearance), extremities, and buttocks, in addition to alopecia and nail dystrophy. On rare occasions, patients can also develop osteosarcoma, fibrosarcoma, gastric carcinoma, and cutaneous squamous cell carcinoma.23
HyperpigmentationDiffuse or focal darkening of the skin is a distinctive skin sign with multiple causes, several of which are malignant. These are listed below and summarized in Table 4. Café au lait spots, which are seen in neurofibromatosis type 1, are dealt with in a later section.
Hyperpigmentation as a Sign of Possible Internal Malignancy.
| Ectopic adrenocorticotropic hormone syndrome |
| Hemochromatosis*** |
| POEMS syndrome* |
| Metastatic melanoma*** |
| Malignant acanthosis nigricans* |
| Leukemia and Hodgkin and non-Hodgkin lymphoma*** |
| Melanocytic macules and papules in the perianal and genital areas*** |
| Cronkhite-Canada syndrome** |
| Peutz-Jeghers syndrome** |
Abbreviation: POEMS, Polyneuropathy, Organomegaly, Endocrinopathy, Monoclonal gammopathy, and Skin changes.
Ectopic ACTH syndrome (**) is a feature of Cushing syndrome caused by the secretion of ACTH by pituitary or ectopic tumors. The hyperpigmentation in such cases is diffuse, similar to that seen in Addison disease, and more intense in exposed areas (face, neck, back of hands), areas affected by trauma or subject to slight pressure, and mucous membranes. The cause is unclear, but the pigmentary changes have been linked to the production of the polypeptide β-lipotropin, which is capable of inducing the production of MSHs, which in turn, stimulate the production of melanocytes in the skin.
ACTH syndrome tends to be associated with the following tumors: small-cell lung carcinoma, carcinoid tumors, pheochromocytoma, medullary thyroid cancer, and reproductive organ and pancreatic tumors. Elevated plasma cortisol and corticotropin levels are highly suggestive of a diagnosis of ACTH syndrome.7,23–25
Diffuse hyperpigmentation is also a feature of hemochromatosis (bronze diabetes) (***), which manifests with hyperpigmentation in advanced states (now quite rare), diabetes mellitus, and hepatic cirrhosis, Hemochromatosis is a risk factor for liver cancer.
Hyperpigmentation is also a feature of POEMS syndrome (*), which is named after some of its main features: Polyneuropathy, Organomegaly, Endocrinopathy, Monoclonal gammopathy, and Skin changes. It is also known as sclerotic myeloma. In this syndrome, hyperpigmentation is normally diffuse and homogeneous, but it can affect acral sites and the trunk. Other cutaneous manifestations are hemangiomas, hypertrichosis, digital clubbing, leukonychia, vasculitis, skin thickening, and scleroderma-like skin changes.26
Late-stage metastatic melanoma (***) is, on rare occasions, associated with diffuse melanosis, which is characterized by a blue-gray color and believed to be caused by tumor lysis. Vitiligo-like depigmentation due to the immune response mounted against melanocytes is not uncommon in this form of melanoma.
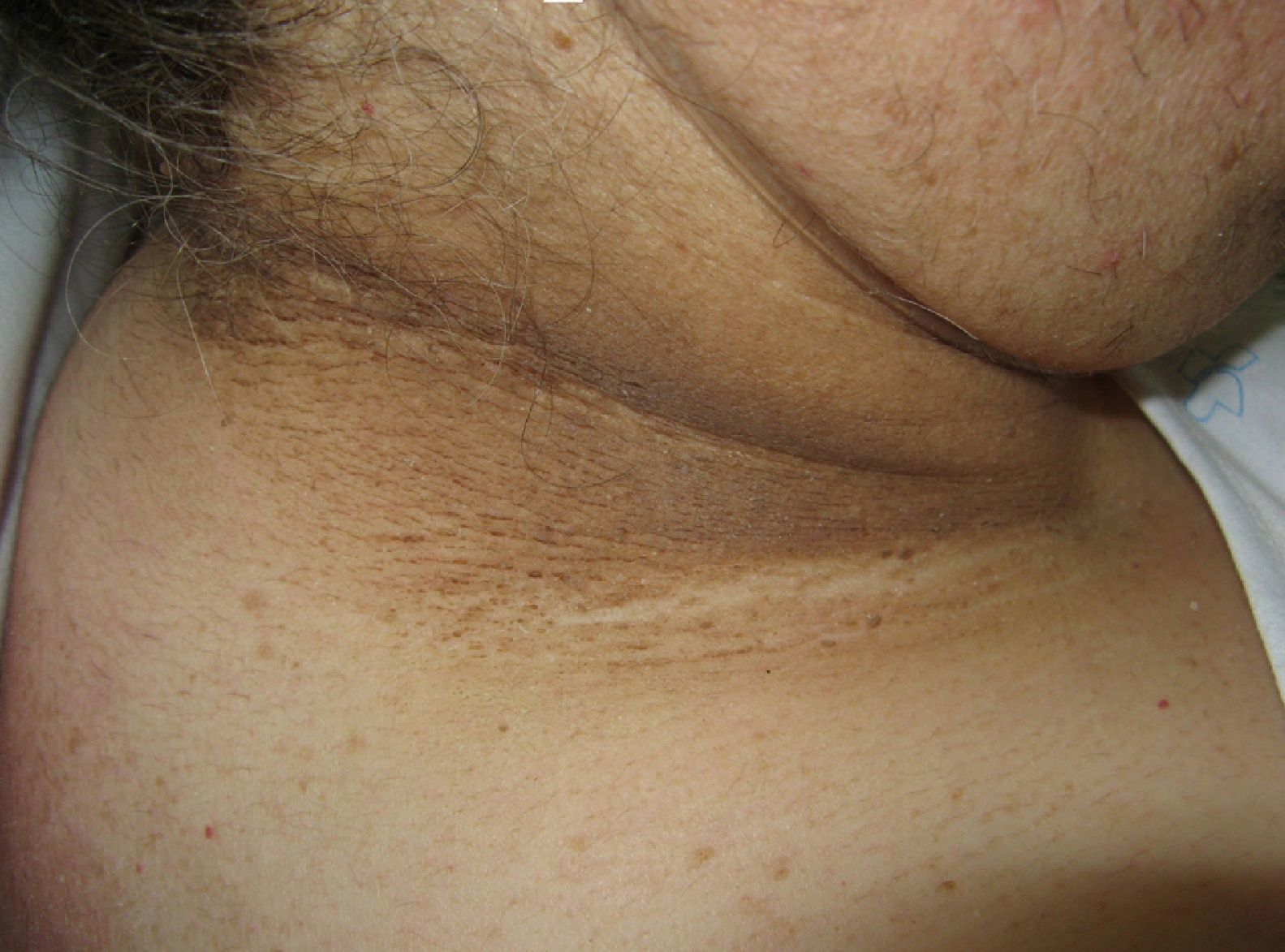
Hyperpigmentation of the skinfolds is generally the first sign of malignant acanthosis nigricans (*).27 Subsequently, the skin acquires a velvety appearance with verrucous lesions affecting the axillae, neck, and inguinal regions (Fig. 4). As the disease progresses, lesions appear on the oral mucosa, the lips, and the areola, and in areas subject to friction. Palmoplantar hyperkeratosis is seen in more advanced cases.
Tripe palms, which are similar in appearance to the lining of bovine, porcine, or sheep stomach, are characterized by a rough velvety appearance with exaggerated dermatoglyphics. The condition is also known as acanthosis palmaris and acanthosis nigricans of the palms. It is considered to be a variant of malignant acanthosis nigricans rather than a separate entity. Patients with this condition have darker-than-normal palms.28
The malignancy most frequently associated with malignant acanthosis nigricans is gastric adenocarcinoma, which may not be diagnosed until several years later; this tumor is thought to possibly secrete growth factors that cause malignant nigricans acanthosis. Less commonly, the condition may also be seen in adenocarcinoma of the uterus, liver, bowels, ovary, kidneys, breast, thyroid, and lungs.27,29
Malignant acanthosis nigricans is also occasionally observed in association with other paraneoplastic conditions, such as eruptive seborrheic keratosis (sign of Lesar-Trélat) and florid cutaneous papillomatosis (development of warty lesions).30,31 It is considered a cutaneous marker of an internal malignancy as it develops in parallel to the growth of the tumor.
It should be recalled that acanthosis nigricans can also occur in benign conditions, such as obesity, endocrine disorders, congenital diseases, or conditions related to the use of medication (nicotinic acid and corticosteroids). Benign acanthosis nigricans appears in childhood or puberty, tends to be unilateral, and has a genetic component.3,20,23,25,32
On occasions, hyperpigmentation is a nonspecific cutaneous manifestation of leukemia and Hodgkin and non-Hodgkin lymphoma) (***); it can also indicate mycosis fungoides, but less frequently. Its pathogenesis is believed to be associated with the secretion of factors stimulating the production of melanin by mast cells, fibroblasts, keratinocytes, endothelial cells, and lymphoid tumor cells.34
Melanocytic macules and papules in the perianal and genital regions have been reported in association with esophageal and lung adenocarcinoma.33,34
Cronkhite-Canada syndrome (**) was first described in 1955 in 2 patients with gastrointestinal dysfunction, pigmentary changes in the form of hyperpigmented lentiginous macules, ranging from a few millimeters in size to 10cm, located on the palms, arms, neck, and scalp in association with alopecia and atrophy of the toenails and fingernails. The syndrome is more common in men and is characterized by diarrhea, weight loss, and neurologic symptoms; 14% of patients develop gastrointestinal carcinoma, but despite its similarities to Peutz-Jeghers syndrome, Cronkhite-Canada syndrome does not have a genetic component.23,24,35
Peutz-Jeghers syndrome (**) is an autosomal dominant disorder characterized by periorificial pigmented macules that are highly evident around the mouth and on the lips, nose, mucous membranes, and fingertips. Patients develop gastrointestinal polyps and hamartomas and have a higher risk of pancreatic, breast, gastrointestinal, cervical, ovarian, and testicular cancer than the general population.23
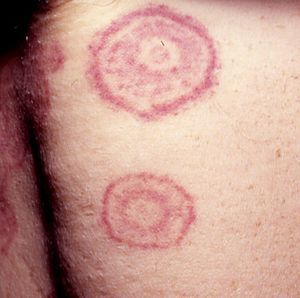
Annular LesionsAnnular lesions are common in a wide spectrum of skin disorders, but in this section, we are going to focus on those lesions that can occur in association with systemic malignancies. We will look at classic paraneoplastic syndromes, such as erythema gyratum repens and migratory necrolytic erythema, as well as at lesions that do not necessarily indicate malignancy but, in certain circumstances, should prompt the clinician to consider the possibility. This second group includes erythema annulare centrifugum, granuloma annulare, subacute cutaneous lupus erythematosus, reticular erythematous mucinosis, and pityriasis rotunda (Table 5).
Skin Disorders With Annular Lesions Potentially Associated With an Internal Malignancy.
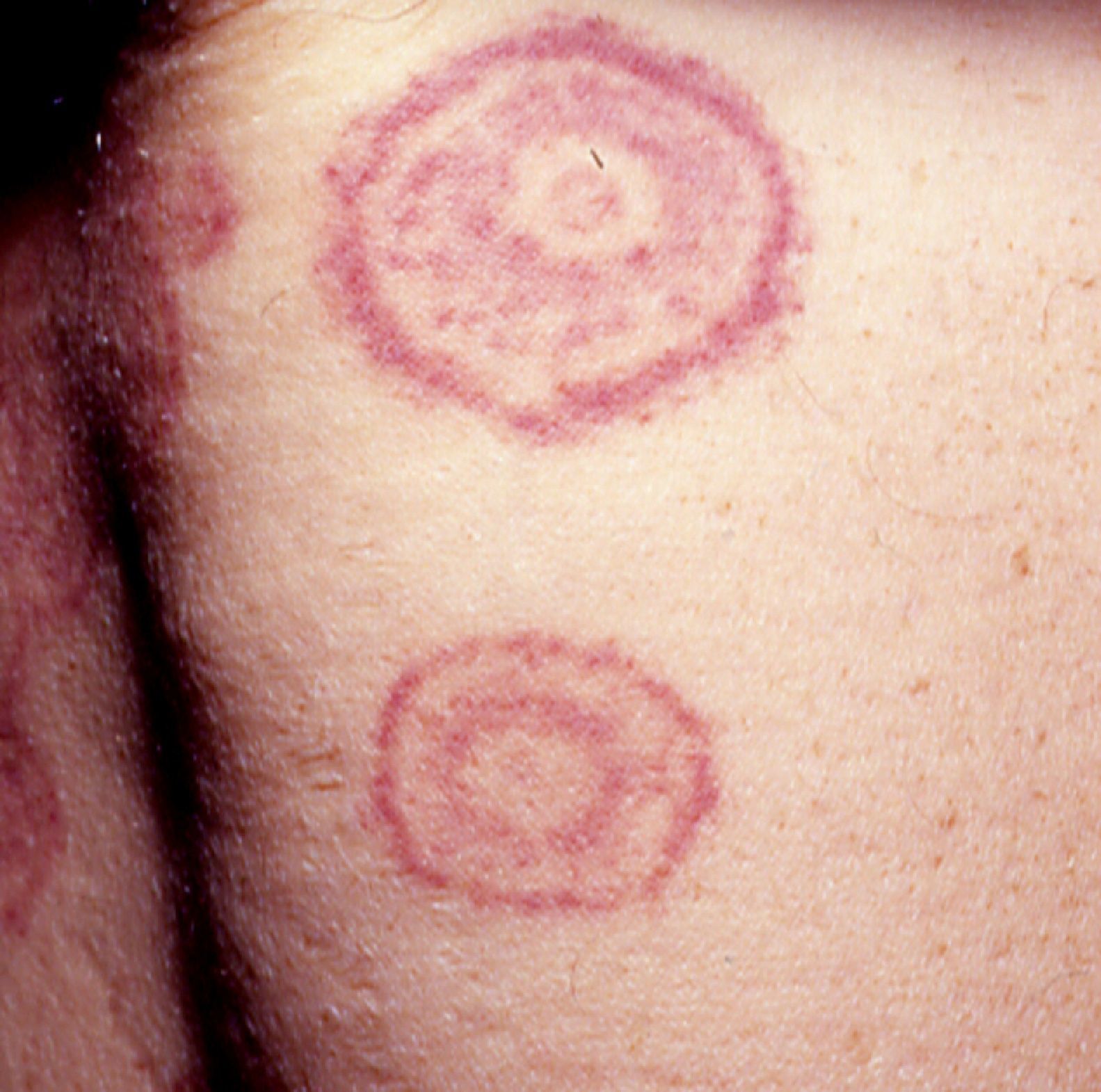
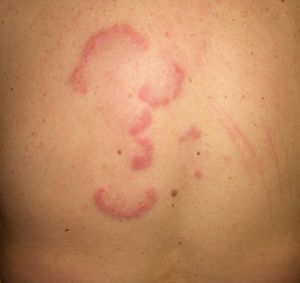
Erythema gyratum repens is the most common type of erythema associated with cancer. It was described by Gammel in 1952 and is associated with an internal malignancy in an estimated 77% to 82% of cases. It is thought to be due to an immune response. It is a rare condition characterized by erythematous, pruritic, serpiginous, concentric rings that advance at a rate of 1cm a day, producing a wood-grain effect on the skin (Fig. 5). It occurs on the trunk and proximal parts of the extremities. Accompanying skin manifestations include palmoplantar hyperkeratosis, nail dystrophy, and ichthyosis.36–39
Erythema gyratum repens is associated with cancer of the lung (32%-40% of cases), the esophagus (8%), and the breast (6%), as well as with cancers of the digestive tract, bladder, uterus, prostate, anus, and oral cavity and pharynx, and with multiple myeloma and malignant melanoma. It generally appears between a month and 6 years before the malignancy is diagnosed, but it can also appear at the same time or even later.
On rare occasions, erythema gyratum repens has also been observed in benign conditions, such as tuberculosis, calcinosis, and CREST (Calcinosis, Raynaud phenomenon, Esophageal dysmotility, Sclerodactyly, and Telangiectasia) syndrome.
Histologic features include moderate spongiosis, parakeratosis, and a perivascular lymphohistiocytic infiltrate. As also occurs with pemphigus, deposits of immunoglobulin G and/or C3 may be seen in the basal membrane.
Erythema gyratum repens responds to treatment of the underlying tumor, and when this is not possible, systemic corticosteroids are useful.2,3,14,20,23,25,38,39
Migratory Necrolytic Erythema (*)Migratory necrolytic erythema is typically associated with glucagon-secreting alpha-islet cell tumors of the pancreas, which cause glucagonoma syndrome. It is characterized by erythematous annular or arciform lesions that tend to coalesce into plaques, adopting a polycyclic pattern. The lesions are distributed in the perioral region, in the distal parts of the extremities, and on the abdomen, perineum, thighs, buttocks, and groin. Blisters, erosions, and crusts are seen at the active, serpiginous borders of lesions. The skin has a dry, cracked appearance that is reminiscent of staphylococcal scalded skin syndrome and acrodermatitis enteropathica (which is due to zinc deficiency). Mucosal involvement causes glossitis and excess glucagon leads to weight loss, glucose intolerance, anemia, diarrhea, alopecia, thromboembolic disease, and psychiatric disorders.
Histologic features include psoriasiform hyperplasia or vacuolization and epidermal necrosis, depending on the stage of disease at the time of biopsy.
Deficient levels of zinc, amino acids, and essential fatty acids have been implicated in the pathogenesis of migratory necrolytic erythema, explaining why this eruption is seen in malabsorption syndromes, liver failure, inflammatory bowel disease, and celiac disease in the absence of glucagonoma. It has also been reported in association with myelodysplastic syndrome without glucagonoma, hepatocellular carcinoma, bronchial carcinoma, and jejunal adenocarcinoma.37–40 Treatment is targeted at the underlying condition, and patients do not respond to zinc supplements. There have been recent reports of the use of octreotide, a somatostatin analog that inhibits glucagon production but does not stop tumor growth.2,3,6,7,23–25,38
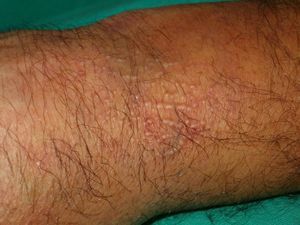
Other Annular LesionsErythema annulare centrifugum (***) (Fig. 6) is believed to be a hypersensitivity reaction to a wide variety of antigens, such as those associated with infections, drugs, and endocrine disorders. It is, albeit very rarely, seen in neoplastic disorders, such as lymphomas, leukemias, and malignant histiocytosis,2,23,38 and there have also been anecdotal reports of an association with prostate cancer.41
Generalized granuloma annulare (***) is a chronic skin disorder of unknown etiology. A delayed hypersensitivity reaction to an unknown antigen has been implicated in its pathogenesis. Factors that induce or are associated with granuloma annulare are trauma, insect bites, tuberculin testing, psoralen-UV-A therapy, viral infection, and diabetes mellitus. There have also been rare reports of an association with Hodgkin lymphoma and even rarer reports of one with other types of solid tumors, such as cancer of the lung, the colon, the cervix, the prostate, the stomach, and the ovary.
When granuloma annulare occurs with an underlying malignancy, it presents an atypical clinical pattern, with painful lesions in uncommon locations, such as the palms and the soles. It affects older patients, with a peak age of onset of 54 years. The erythematous eruption can precede the detection of an underlying tumor by 18 months or may not appear until up to 7 years later. Middle-aged patients with persistent generalized granuloma annulare that responds poorly to treatment should undergo screening for an underlying tumor.42,43
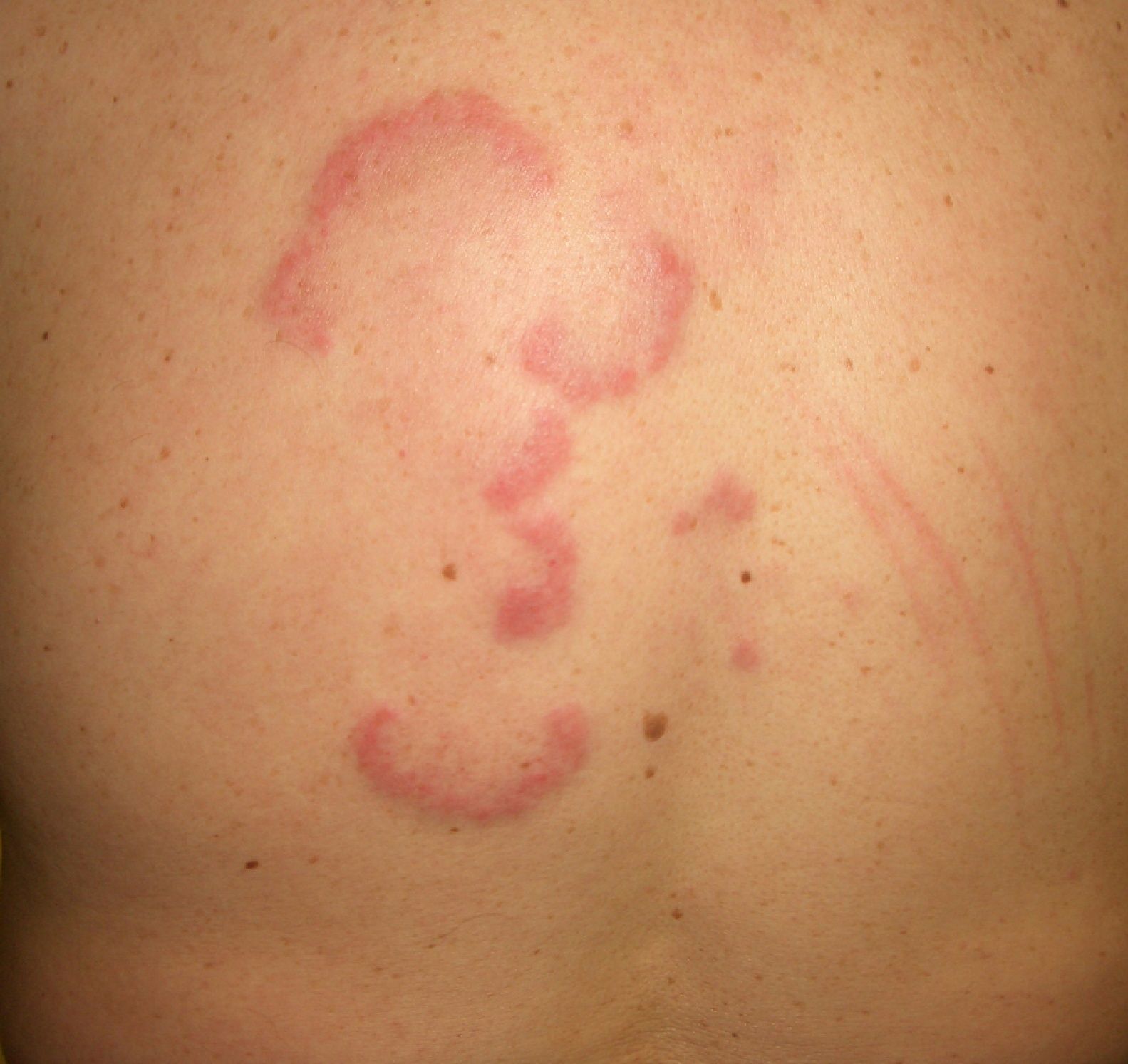
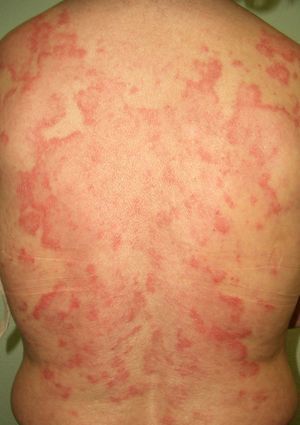
The association between acute cutaneous lupus erythematosus (***) (Fig. 7) and systemic malignancy was first described in 1980, and in total, approximately 14 cases have been described in association with lymphoma and cancer of the lung, breast, stomach, uterus, liver, and larynx. The lesions predate the diagnosis of the underlying tumor by between a month and 3 years. Despite these reports, however, doubts still remain about the paraneoplastic nature of granuloma annulare; the possibility of an underlying malignancy may be considered in cases with a clear temporal relationship, and screening studies are an option in cases that are refractory to treatment.44–46
Reticular erythematous mucinosis (***) is a primary cutaneous mucinosis characterized by reticular pruritic, erythematous lesions on the upper chest. It rarely occurs in association with malignancy, but has been described in patients with cancer of the colon, breast, and lung.47
Pityriasis rotunda (***) is a rare disease characterized by well-delimited circular, scaling lesions, which may be lighter or darker than the surrounding skin. Patients develop multiple lesions on the trunk that may coalesce. There is no associated inflammation or pruritus. Some authors believe it to be a localized form of acquired ichthyosis. Pityriasis rotunda is more common in Japanese individuals, but it has been described in other populations. It can occur in association with tuberculosis, leprosy, and lung or liver disorders.
Associated malignancies include hepatocellular carcinoma, cancer of the stomach, esophagus, palate, and prostate, and chronic lymphoid leukemia and multiple myeloma.6,25
Erythematosquamous and Hyperkeratotic LesionsLesions with a chronic eczematous or psoriasiform appearance are a common cause of consultation and in most cases do not require further investigation. In some cases, however, an occult malignancy may be present and such a possibility should be ruled out in the event of a high index of clinical suspicion (Table 6).
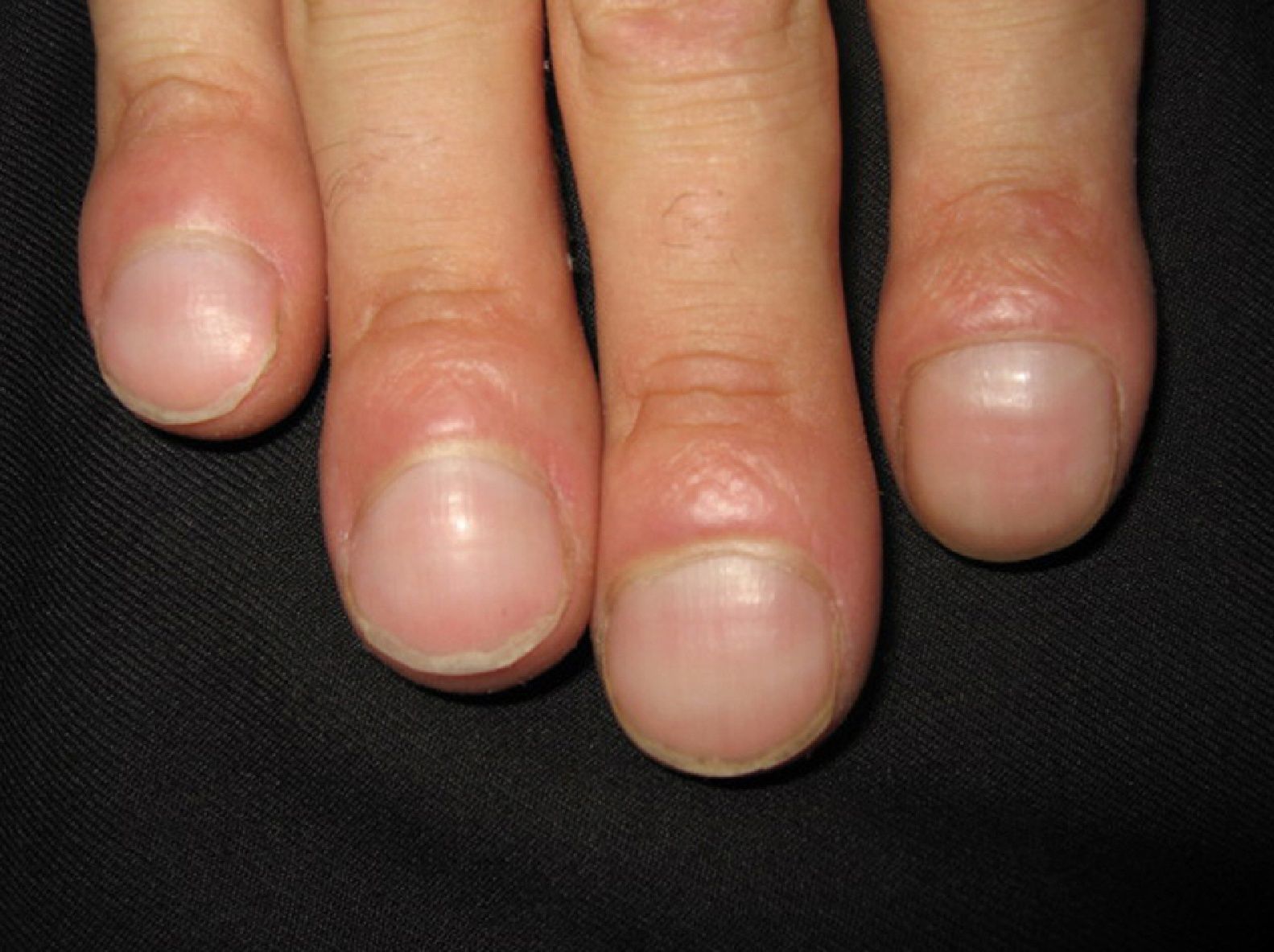
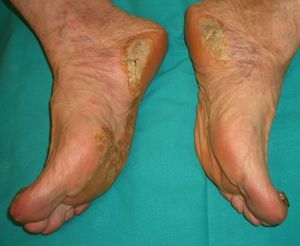
The first entity discussed in this section is acrokeratosis paraneoplastica of Bazex (Bazex syndrome)(*), which was first described in 1965. This is a psoriasiform condition that affects men between 60 and 70 years of age. It presents with symmetric erythematous, scaling lesions that affect the hands, the feet, the elbows, and the knees. As the condition progresses, patients develop palmoplantar keratoderma with a violaceous color and marked nail dystrophy (Fig. 8), followed by psoriasiform lesions on the ears, the nose, the trunk, and the scalp. The lesions do not respond to conventional treatment. Histologic features differ to those seen in psoriasis, and consist of hyperkeratosis with focal parakeratosis, acanthosis, dyskeratotic keratinocytes, and a lymphocytic perivascular infiltrate.
Psoriasis must be considered in the differential diagnosis; the lesions have a psoriasiform appearance, but they are poorly circumscribed and have a fine pityriasiform scale and a blue color; vesicles and blisters may also develop. Palmoplantar hyperkeratosis is more common at the pressure points of the palm and does not affect the central area.
The lesions can precede the detection of the underlying malignancy by a year; commonly associated malignancies are squamous cell carcinomas of the larynx, esophagus, and lung,48,49 but some cases have been reported in other malignancies, including ductal breast adenocarcinoma, cholangiocarcinoma, adenocarcinoma of the colon and prostate,50 lymph node metastasis in the neck, and liposarcoma.51
Several factors have been implicated in the pathogenesis of acrokeratosis paraneoplastica of Bazex, including antigen cross-reactivity between the tumor and the skin, production of a keratinocyte growth factor, vitamin A deficiency, and genetic susceptibility. Prognosis can be improved by conducting an exhaustive investigation for an occult malignancy.2,3,20,23–25,32
There have also been anecdotal reports of other erythematosquamous lesions occurring in association with malignant tumors; these lesions include small plaque parapsoriasis (***), described in a liposarcoma,52 and pityriasis rubra pilaris (***), described in Merkel cell carcinoma cutaneous squamous cell carcinoma, hepatocellular carcinoma, breast carcinoma, and lung carcinoma.53 Some authors have suggested that disseminated superficial porokeratosis (***) might be a paraneoplastic presentation in hematologic malignancies, hepatocellular carcinoma, cholangiocarcinoma, and esophageal and ovarian carcinoma.54–57
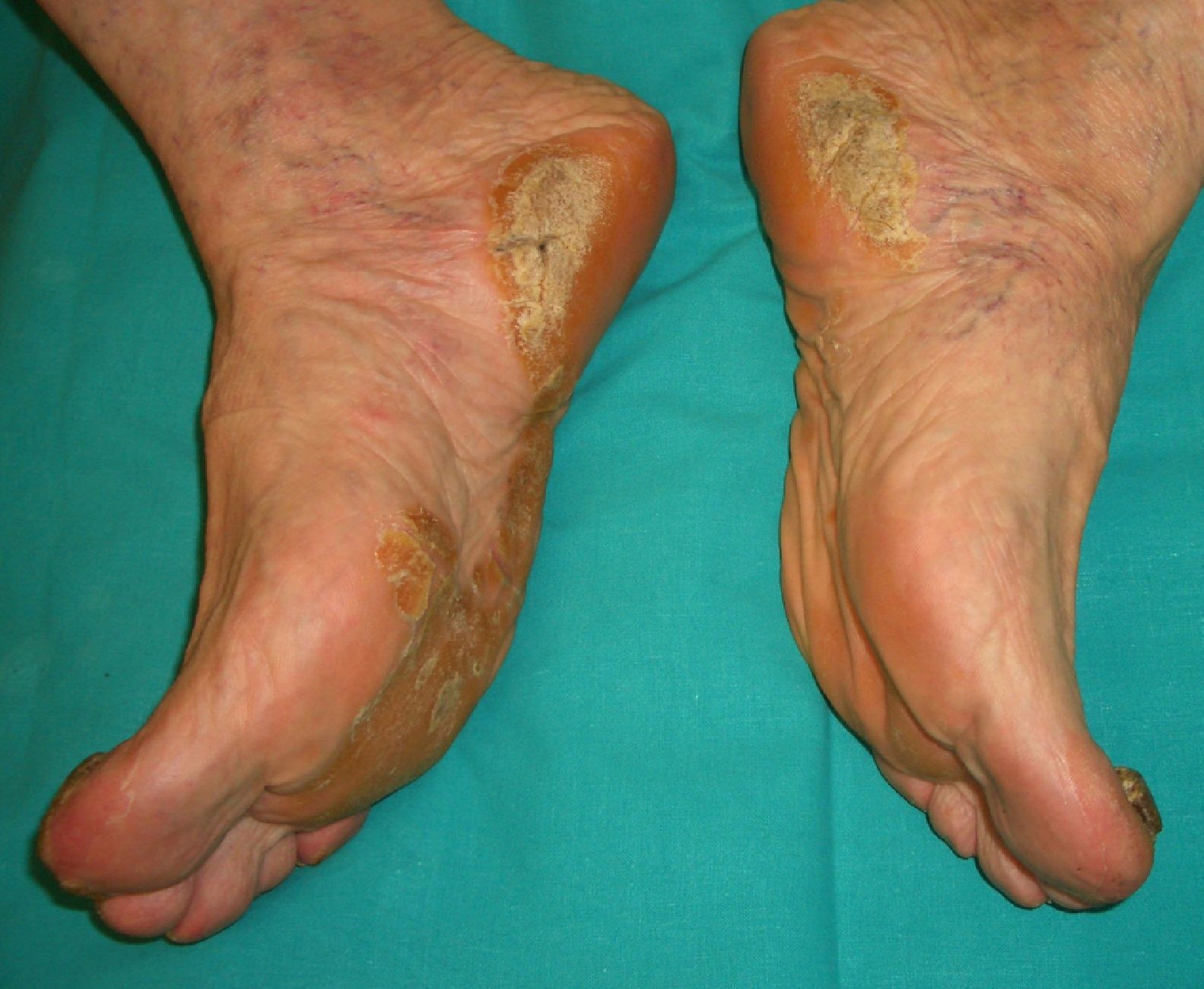
Palmoplantar keratoderma (**) is seen in benign conditions, such as psoriasis, pityriasis rubra pilaris, climacteric, or as mentioned earlier, Bazex syndrome, acanthosis nigricans (tripe palms) erythema gyratum repens, and others.
There is a hereditary form, however, known as Howell-Evans syndrome, that is associated with a higher incidence of esophageal cancer. Palmoplantar keratosis may also occur in chronic arsenic poisoning, which is a risk factor for several cancers.
The sudden onset of palmoplantar keratoderma in the absence of other findings should prompt investigation of an underlying malignancy. The keratoderma in such cases may be punctate-type, focal, or diffuse and there may or may not be hyperhidrosis (Fig. 9). Prognosis is poor and the condition can indicate lung or esophageal cancer, or, less commonly, breast or pancreatic cancer or myeloma.2,20,32,58
Skin Thickening and HardeningIn certain conditions, the skin can gradually become thicker and harder than normal, leading to changes in physical appearance. Such changes occur in storage disorders, such as amyloidosis and scleromyxedema, or in disorders such as scleroderma or scleredema in which progressive hardening is observed. On other occasions, they are due to excessive growth or to the thickening of all the layers of the skin, seen in conditions such as pachydermoperiostosis.
The above manifestations are all paraneoplastic, although on many occasions, they are benign and have no association with malignancy (Table 7).
Primary systemic amyloidosis is caused by the extracellular accumulation of an insoluble fibrillar protein in tissues throughout the body. The condition can be diagnosed histologically on detection of green birefringence when tissue stained with Congo red is examined under polarized light. Aspiration of subcutaneous abdominal fat is the preferred means of obtaining tissue for examination.
Amyloidosis is classified as primary amyloidosis, which includes an idiopathic form and a form associated with multiple myeloma, or secondary amyloidosis, which is due to chronic inflammatory disease.
The skin is affected in 25% of cases of primary systemic amyloidosis. Patients develop waxy skin and pinch purpura, which refers to the appearance of purpuric lesions following minimal trauma. This purple coloring is more noticeable around the eyes and on the face and neck (Fig. 10). Papules, plaques, blisters, nodules, alopecia, and scleroderma-like manifestations may also develop. Infiltration of the tongue can cause macroglossia. The involvement of organs other than the skin can lead to heart failure, Carpal tunnel syndrome, polyneuropathy, kidney failure, and other problems.
Primary systemic amyloidosis is considered to be paraneoplastic because it occurs in 15% of multiple myelomas.3,24,25,59,60
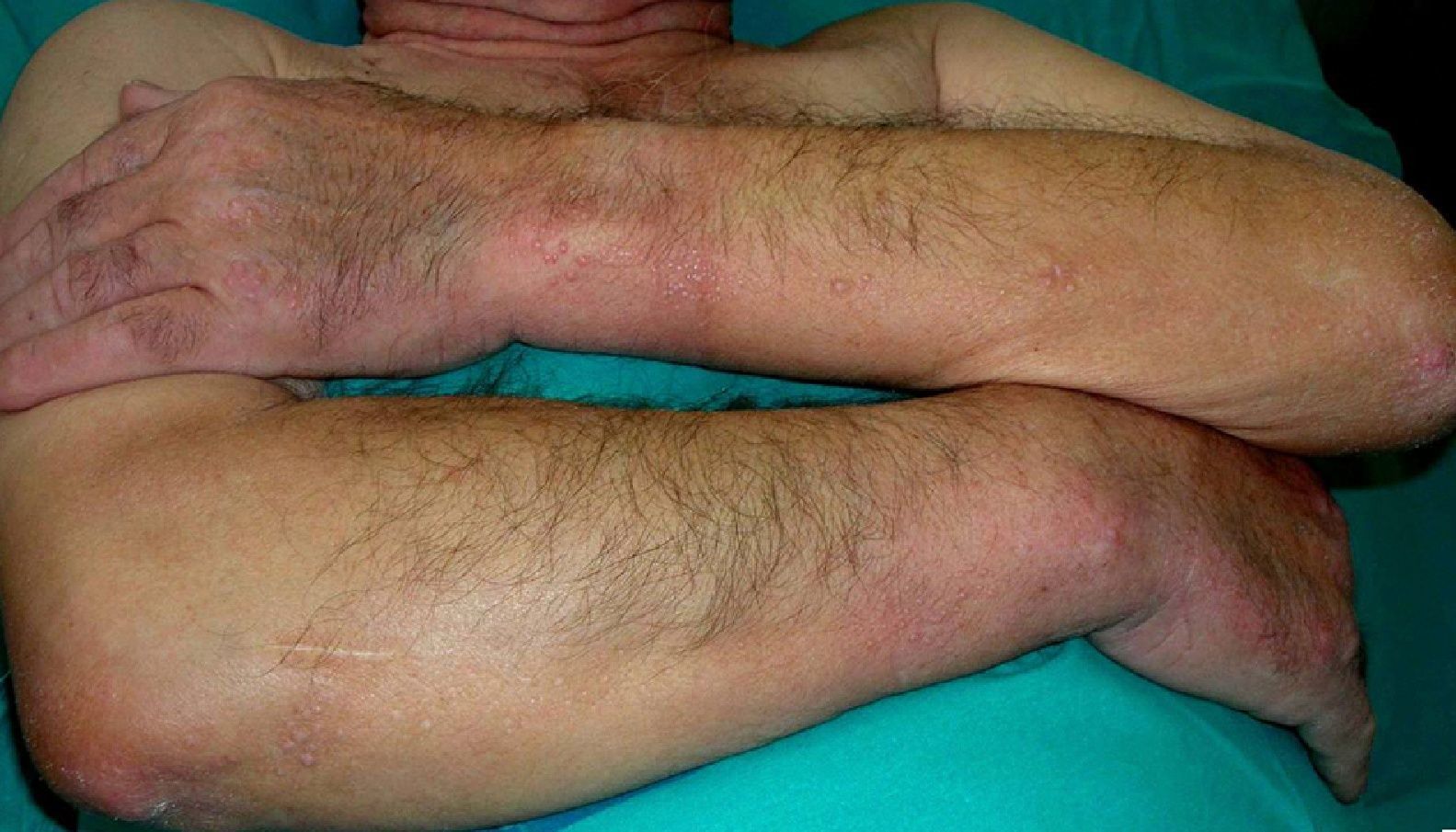
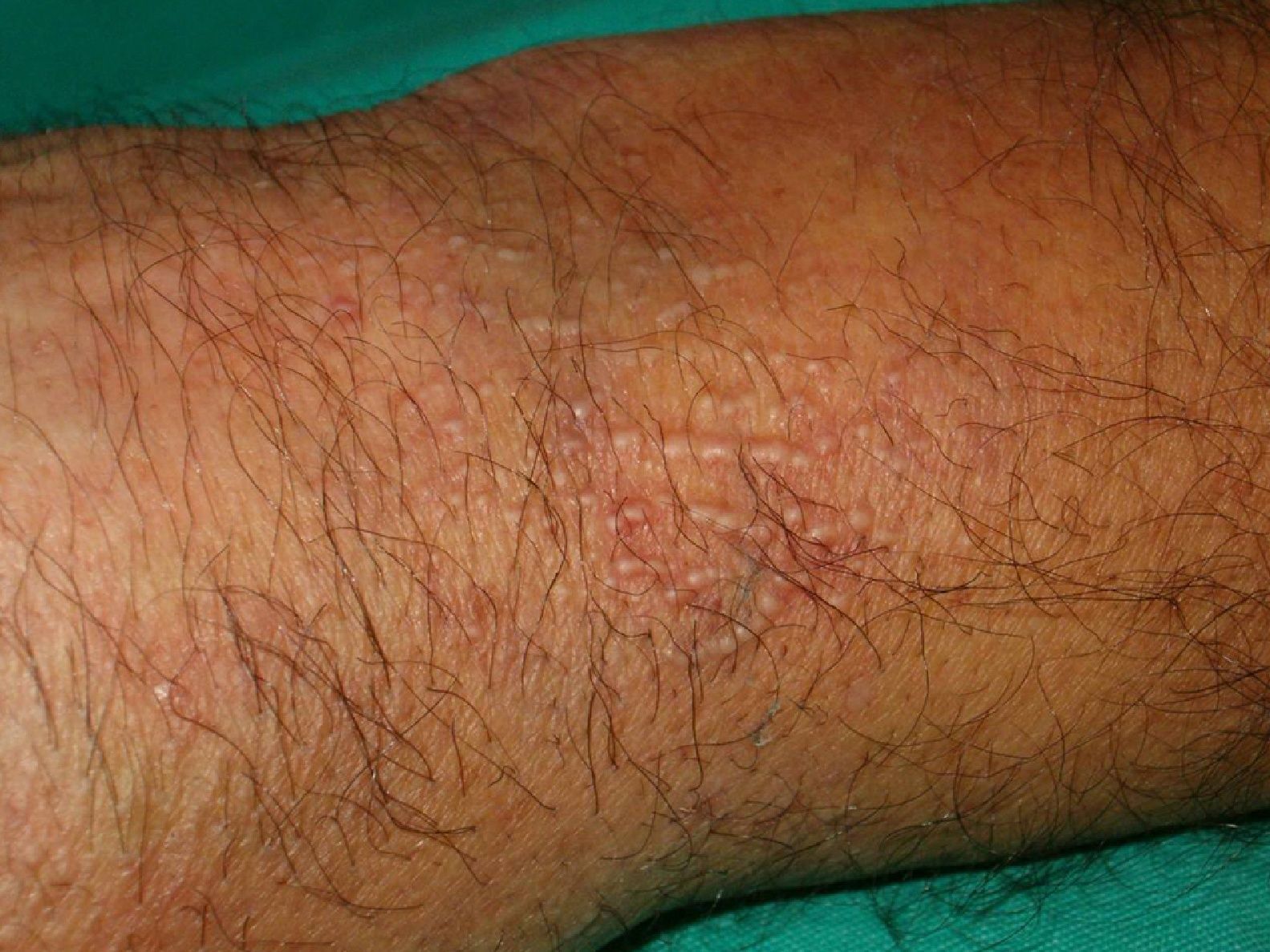
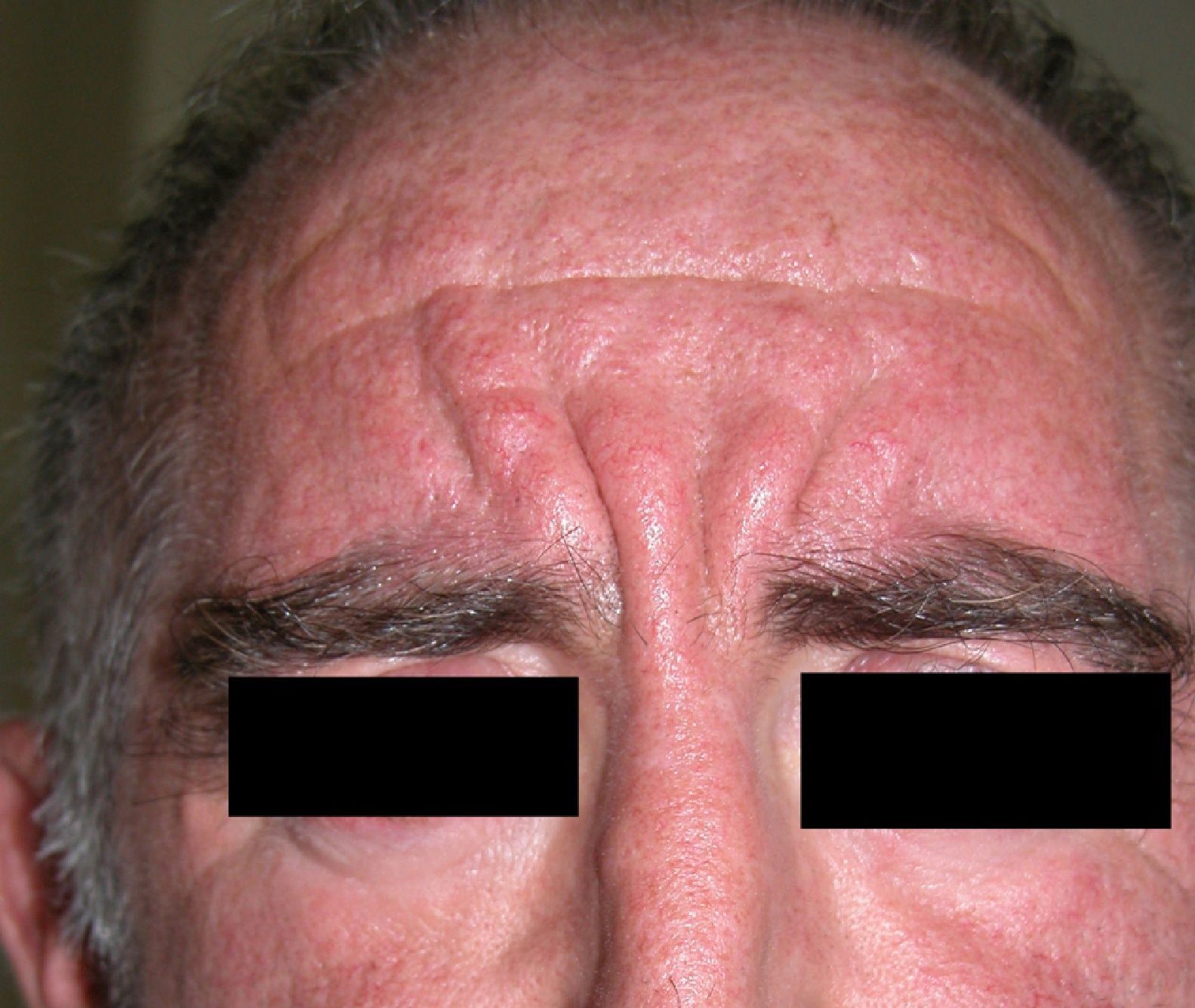
Scleromyxedema (**)Scleromyxedema is the diffuse variant of lichen myxedematosus. It consists of millimetric waxy papules that form clusters of linear, symmetrically distributed lesions on the hands, the elbows, the forearms, the trunk, the face, and the neck (Figs. 11 and 12). The papules coalesce, giving the skin a thick, scleroderma-like appearance and resulting in decreased joint motility. The furrowing of the skin on the face can result in leonine facies and difficulties with facial expression. Systemic manifestations include dysphagia, proximal muscle weakness, peripheral neuropathy, Carpal tunnel syndrome, and kidney disorders.
Histology shows a proliferation of fibroblasts, fibrosis, and dermal mucin deposits. Scleromyxedema is associated with monoclonal gammopathy in 80% of cases. Less commonly, it is seen in patients with multiple myeloma, Waldenström macroglobulinemia, Hodgkin and non-Hodgkin lymphoma, and leukemia. It can also indicate POEMS syndrome26 or thymic carcinoma.61
Scleromyxedema runs a chronic, progressive course, with skin manifestations preceding systemic involvement.3,22,25,59,62
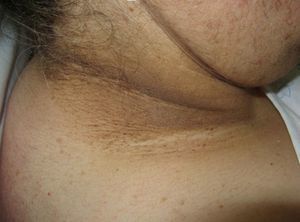
Scleredema (**)Scleredema adultorum of Buschke commonly occurs in association with diabetes mellitus, but it has also been described in lymphomas and myeloma. It starts with nonpitting hardening and swelling of the neck and upper chest and should be clinically distinguished from scleromyxedema and scleroderma. Biopsy shows thickened collagen with mucin in large edematous spaces, but there is no fibroblastic proliferation. It can affect organs other than the skin.25,62
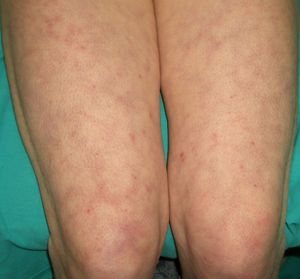
Systemic Sclerosis or Scleroderma (***)Systemic sclerosis, or systemic scleroderma, is a chronic inflammatory autoimmune condition characterized by vascular changes, immune system activation, and fibrosis of the skin and other organs. One of the cutaneous manifestations is progressive hardening of the skin (Fig. 13). The first symptom tends to be Raynaud phenomenon, which reflects the involvement of the small blood vessels.
Retrospective studies have shown that systemic sclerosis is associated with an increased risk of malignancy, in particular lung and breast cancer, and to a lesser extent B-cell lymphoma. Altered B-cell function, which can give rise to lymphoid malignancies, has been implicated in the pathogenesis of this autoimmune disorder.59,63–65
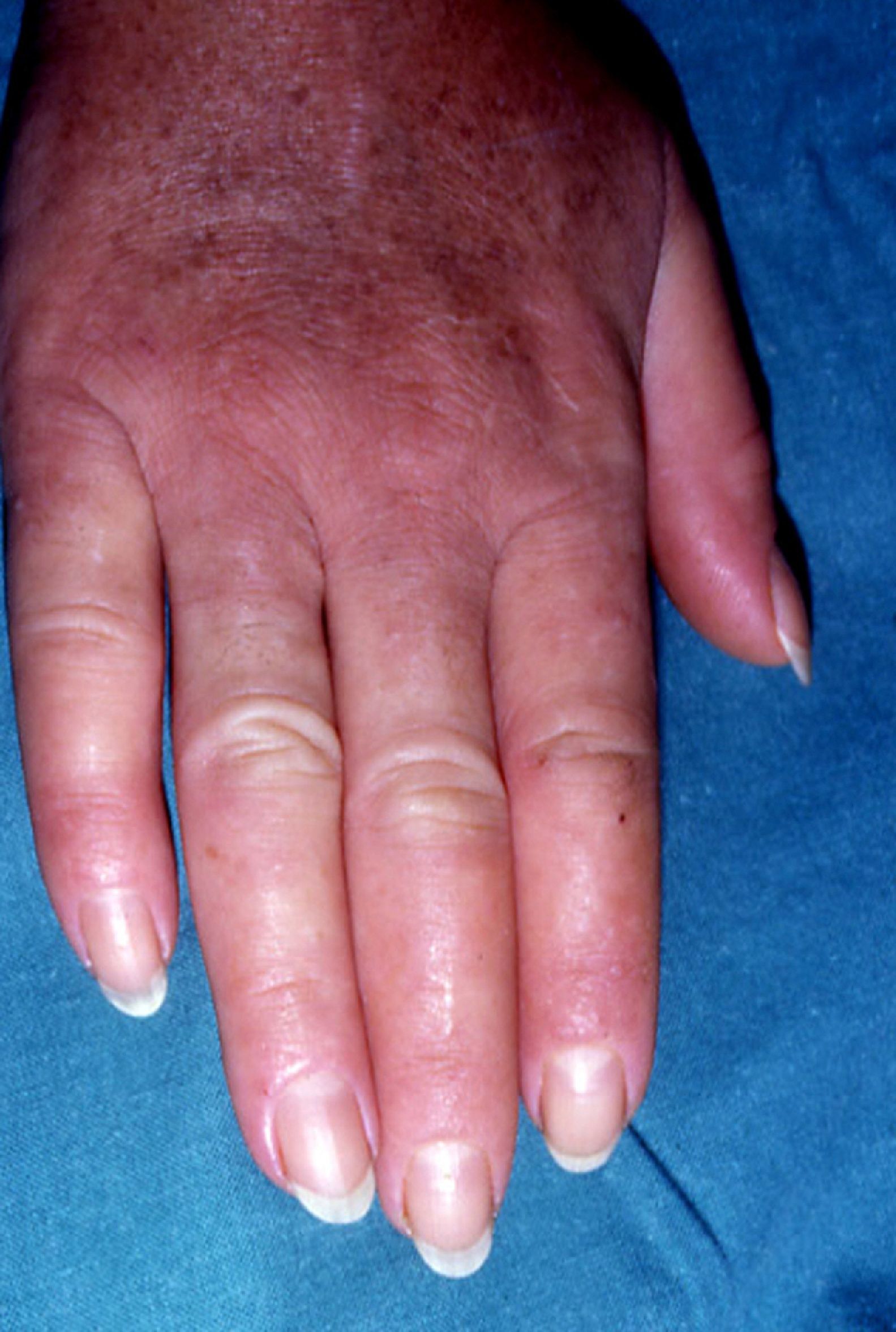
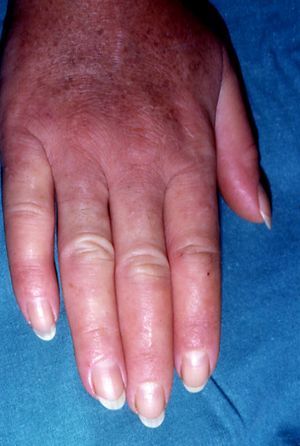
Pachydermoperiostosis or Hypertrophic Osteoarthropathy (**)Pachydermoperiostosis is characterized by digital clubbing (Fig. 14), hypertrophic osteoarthropathy, and cutaneous manifestations including diffuse, progressive thickening of the skin with seborrhea, acne, folliculitis, dilated pores, furrowing of the skin, palmoplantar hyperhidrosis, and decreased facial and pubic hair (Fig. 15). Overall, patients develop coarse, acromegaloid features.
There is a primary and secondary form of the disease. The former is usually hereditary and progresses slowly, while the latter—the paraneoplastic form—is associated with cardiopulmonary disorders. In 80% of cases, the paraneoplastic presentation is associated with lung cancer, metastases to the lung, nasopharyngeal carcinoma, and other less common cancers.19,25,29,66,67
Vascular LesionsVascular skin manifestations in patients with underlying malignancies are quite nonspecific.5 They are many and diverse, and include telangiectasia, purpura, ischemic lesions, vasculitis, thrombophlebitis, livedo reticularis, and palmar erythema (Table 8). Collateral circulation, which is sometimes very evident, has already been discussed in the section on superior vena cava syndrome.
Because these manifestations are often benign and have multiple causes, the possibility of an underlying malignancy should be investigated in atypical cases with an unknown etiology. In such cases, a detailed clinical history is required to establish a logical correlation.
Telangiectasia (***)Clusters of telangiectasias on the anterior surface of the thorax may indicate breast cancer, or reveal the presence of a subcutaneous metastasis. They have been reported in association with carcinoid tumors and hepatic adenocarcinoma.
Generalized telangiectasias can indicate the presence of a malignant angioendotheliomatosis (intravascular lymphoma); in such cases, the vessels overlie specific lesions and are hard and painful.13
In patients with ataxia-telangiectasia (Louis-Bar syndrome), which is a hereditary immunodeficiency disorder characterized by difficulty walking in the second or third year of life, the telangiectasias can appear on the conjunctivae, the eyelids, the ears, and the cheeks. Patients with this syndrome have an increased risk of lymphoid tumors compared with the general population.23
Purpura and Ecchymosis (***)Nonpalpable purpuric lesions and ecchymosis can sometimes reveal thrombocytopenia or idiopathic thrombocytopenic purpura, commonly seen in multiple myeloma, Waldenström macroglobulinemia, lymphoma, chronic lymphoid leukemia, breast cancer, ovarian cancer, cervical cancer, prostate cancer, and undifferentiated lung cancer.
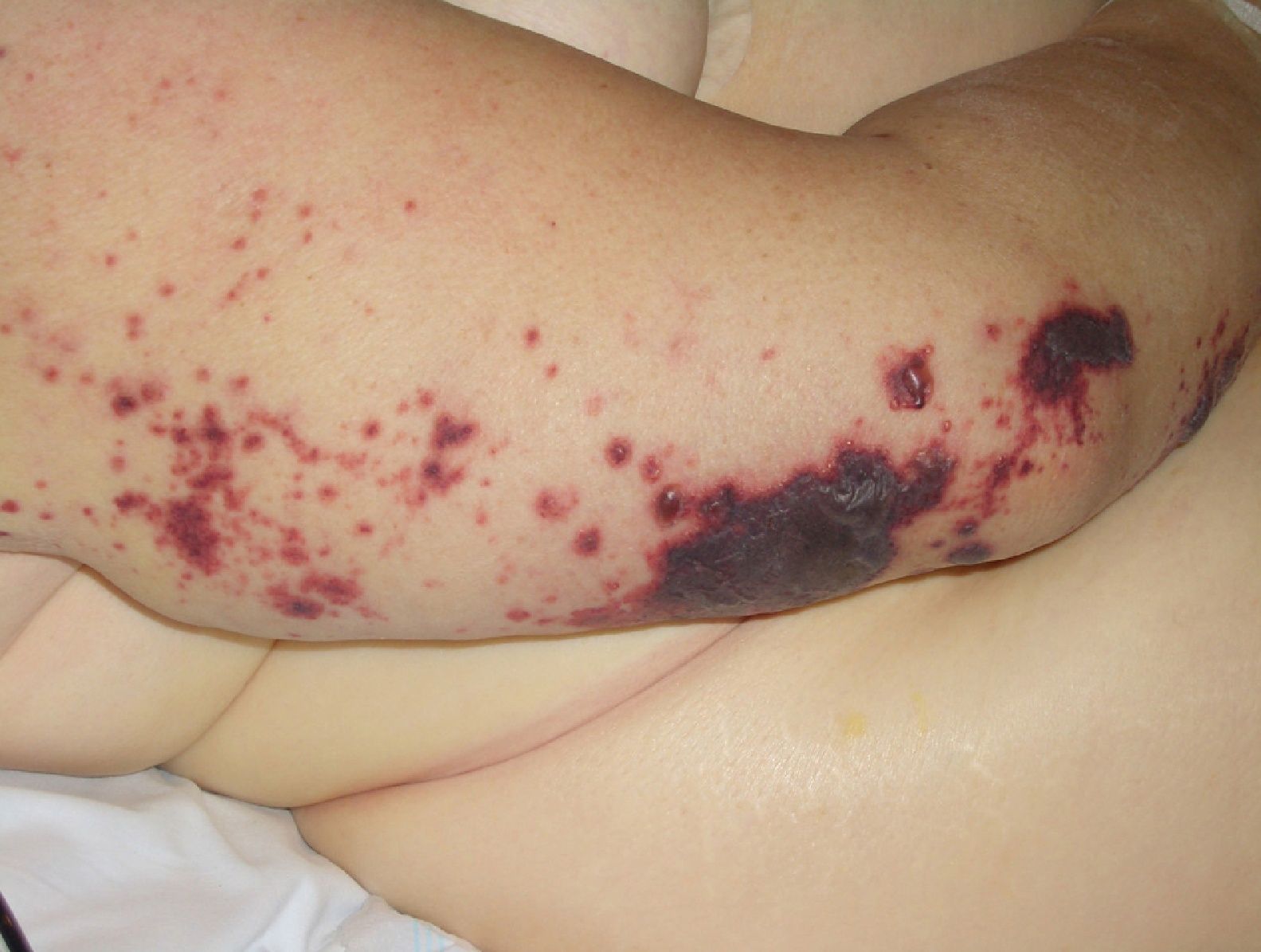
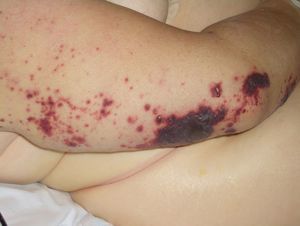
Disseminated intravascular coagulation causes purpura fulminans (Fig. 16) in several malignant hematologic disorders and some solid tumors, such as pancreatic, gastrointestinal, and prostate cancer.
Thrombotic thrombocytopenic purpura is a late sign associated with gastric and breast cancer.
Purpura is also seen in patients with hyperglobulinemia (lymphoma or myeloma). Cryoglobulinemic purpura affects acral sites and occurs in association with Raynaud phenomenon.5,10
Cutaneous Ischemia (***)Digital ischemia is an uncommon paraneoplastic presentation. It can present as a stabbing, burning pain in the feet and warmth of the extremities without any physical signs; it subsequently affects the hands. This condition is known as erythromelalgia and it can precede the diagnosis of a malignancy by more than 2 years. The malignant conditions in which it is seen are due to leukostasis, a known phenomenon in leukemia involving white blood cell counts of over 150 000 cells/mm3 that can cause myocardial infarction and hemorrhaging. Similar manifestations occur in polycythemia vera. Patients may develop skin lesions resembling chilblains.
Peripheral ischemia, which is a paraneoplastic acral vascular syndrome, is also associated with lymphoma and pancreatic, stomach, small-intestine, ovarian, and renal carcinoma.68,69
Ischemia in patients with cryoglobulinemia is caused by increased blood viscosity and is associated with multiple myeloma, Waldenström macroglobulinemia, and lymphoma.13,24
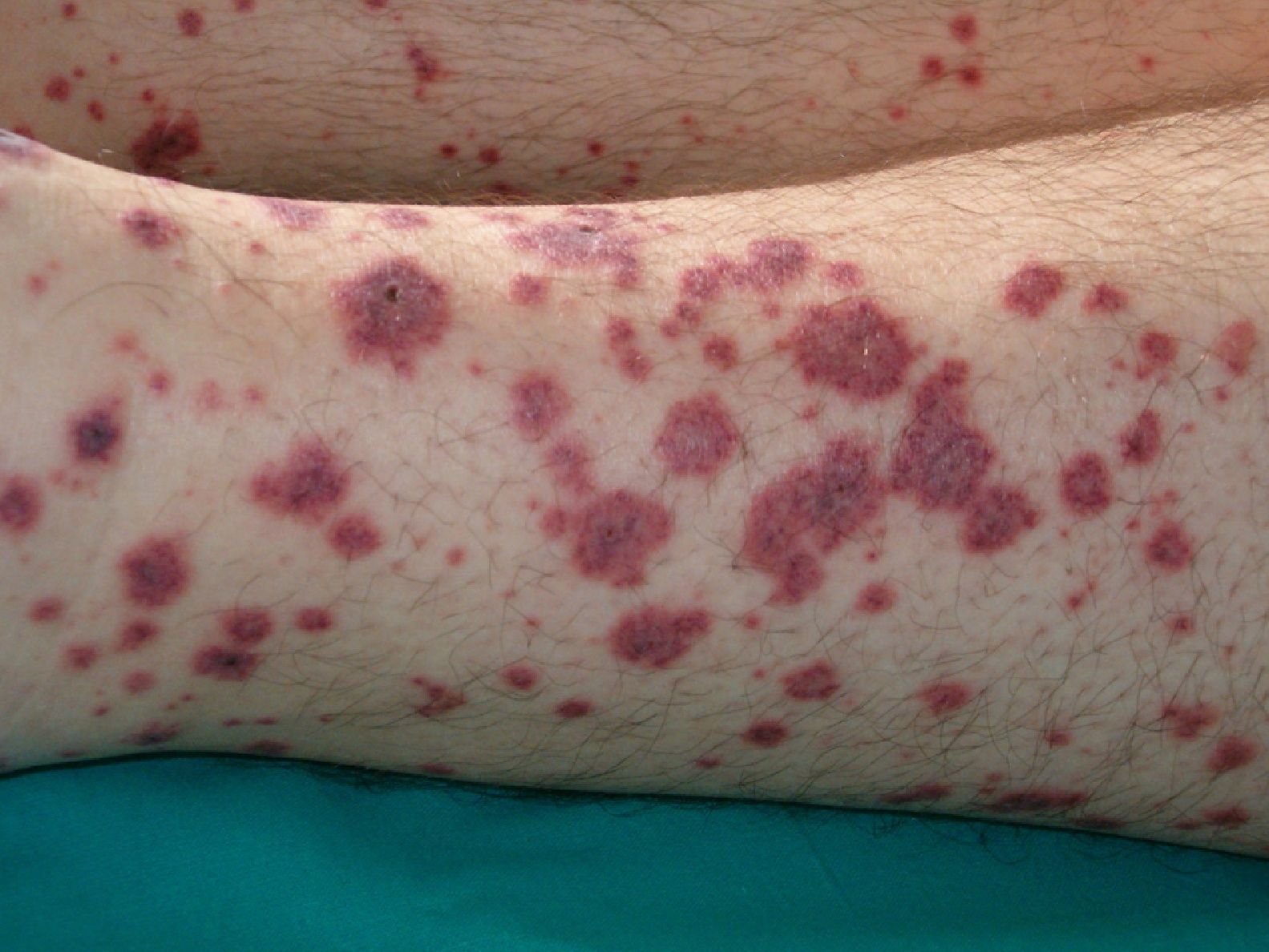
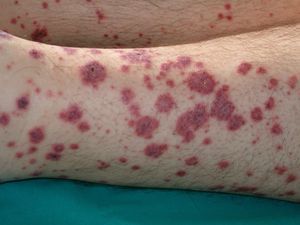
VasculitisVasculitis is associated with malignancies in between 3% and 8% of cases. Almost 80% of patients have an associated hematological malignancy, while the remaining 20% or so have a solid tumor or a tumor of unknown origin. Some tumors are more common in patients with certain subtypes of vasculitis. For example, solid tumors are more frequently associated with Henoch-Schönlein purpura temporal arteritis, while lymphoma and leukemia are more frequently associated with leukocytoclastic cutaneous vasculitis and polyarteritis nodosa. The clinical presentation is heterogeneous, with palpable purpura, papules, nodules, urticaria, necrosis, and ulceration (Fig. 17).2,5
The hematologic malignancies associated with vasculitis include hairy cell leukemia, chronic lymphoid leukemia, myeloma,70 Hodgkin and non-Hodgkin lymphoma, acute myeloblastic leukemia, malignant histiocytosis, and myelodysplastic syndromes.
The most common solid tumor associated with this paraneoplastic presentation is lung cancer, followed by gastrointestinal, renal, urinary, prostate, and breast cancer.71
There are several hypotheses regarding the pathogenesis of vasculitis in such cases, including the formation of antigen-antibody immune complexes associated with the tumor, direct vascular damage to endothelial cells due to a cross reaction with antigens in the tumor cells, and a direct effect of leukemic cells (hairy cells) on the vascular wall.72–75
Vasculitis can predate the diagnosis of the malignant condition by 2 to 4 years. It is therefore important to monitor at-risk patients, i.e. elderly patients with chronic or persistent lesions that respond poorly to treatment.5,71
Urticarial vasculitis has been associated with multiple myeloma, metastatic adenocarcinoma of the colon, metastatic teratoma from a testicular tumor, non-Hodgkin lymphoma, and renal carcinoma.76,77
Thrombophlebitis (**)Venous thrombophlebitis in isolation is rarely a sign of malignancy. However, spontaneous, recurrent, multiple, superficial, or migratory thrombophlebitis (Trousseau syndrome) is paraneoplastic in 50% of lymphomas, leukemias, and cancers of the pancreas, lung, prostate, stomach, and colon. The appearance of this sign is related to a generalized hypercoagulable state.
Patients with cancer have a considerably increased prevalence of deep vein thrombosis. They tend not to have risk factors for deep vein thrombosis, are generally older than 50 years, and respond poorly to anticoagulant treatment. Upper extremity involvement is more common than lower extremity involvement in patients with underlying malignancies. The most common tumors detected are mucin-secreting adenocarcinomas of the gastrointestinal tract, the genitourinary tract, the lung, and the breast.
Thrombophlebitis of the subcutaneous veins of the anterolateral aspect of the chest and abdomen, known as Mondor disease, is usually the result of trauma, physical activity, inflammatory conditions, infections (mastitis, abscesses), or mastoptosis. It is important because in several cases it can play a key role in the diagnosis of breast cancer13,20,23,24; accordingly mammography is indicated in all patients with Mondor disease.
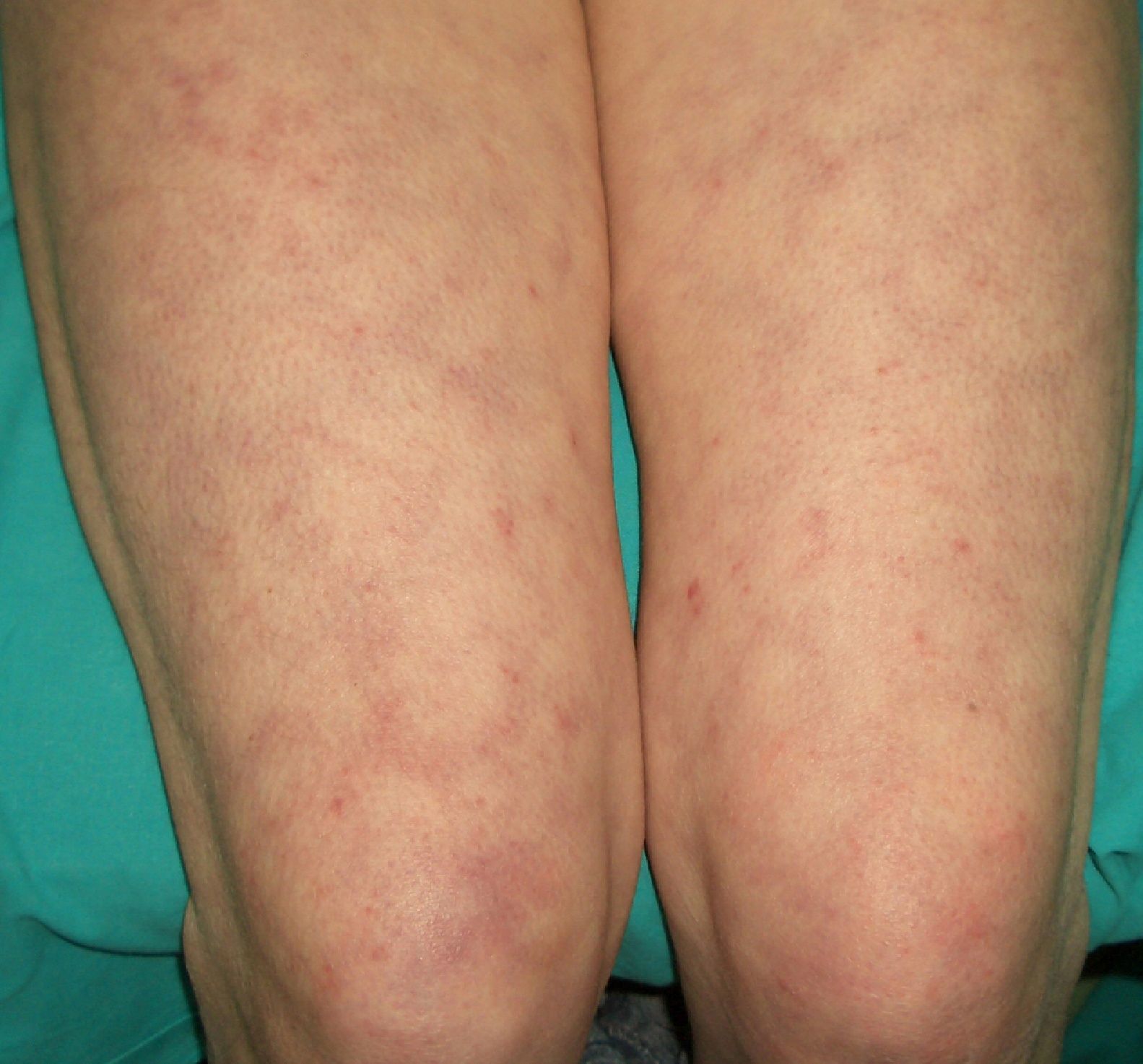
Livedo Reticularis (***)Any conditions that cause increased visibility of the plexus can give rise to livedo reticularis. The main causes are venodilatation and deoxygenation of the blood in the venous plexus (Fig. 18). Livedo reticularis can therefore be caused by vasospasm due to cold or mechanical injury, arterial and venous thrombosis, or increased blood viscosity This explains why patients with malignant conditions, such as multiple myeloma, essential thrombocythemia, and polycythemia may develop livedo reticularis. The condition, though, has also been described in cutaneous T-cell and B-cell lymphoma, angiotropic lymphoma, acute lymphoid leukemia, renal carcinoma, and inflammatory breast carcinoma. The cause is vascular obstruction in most of these cases.78
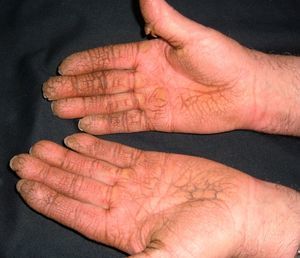
Palmar Erythema (***)Palmar erythema is an acral vascular syndrome whose main causes are pregnancy, liver failure, and rheumatoid arthritis, although it can also occur in healthy individuals. It is attributed to vasodilatation (Fig. 19).
An association has been reported for lung cancer, Hodgkin lymphoma, myeloproliferative syndrome, gastric adenocarcinoma, and brain tumors.79
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Yuste-Chaves M, Unamuno-Pérez P. Alertas cutáneas en malignidades sistémicas (parte I). Actas Dermosifiliogr. 2013;104:285–98.