Bone wax is an inert, malleable material used as a hemostatic agent in treating surgical defects. Healing by secondary intention is an appropriate approach for certain situations in dermatologic surgery. When surgical wounds are deep enough for such tissues as bone or cartilage to be exposed, dressings may adhere to granulation tissue, making removal and subsequent wound care difficult and painful. In such cases bone wax can be molded around deep tissues to create an ideal occlusive, hemostatic microenvironment that facilitates second-intention wound healing.
La cera para huesos es un material inerte y maleable que se utiliza como hemostático en los defectos óseos. La curación por segunda intención es una opción terapéutica en determinados casos después de la cirugía dermatológica. En las heridas quirúrgicas profundas, con exposición de distintos tejidos como hueso o cartílago, ciertos apósitos pueden adherirse al tejido de granulación. Esto dificulta y hace muy dolorosa la curación posterior. En estos casos la aplicación de un molde con cera para huesos proporciona un microambiente oclusivo hemostático ideal que favorece la cicatrización por segunda intención.
In dermatologic surgery wound healing by second intention is an alternative to surgical reconstruction by direct closure, grafts, or flaps. Although with this approach the wound requires more time to heal, it has the oncologic advantage of not involving the displacement of adjacent tissue planes, which can conceal possible deep tumor foci. If the surgical wound is allowed to close completely by second intention, however, there may be complications such as bleeding, pain, and, especially, the contraction and retraction of the wound that can lead to an unsightly scar.
The second-intention healing of any surgical wound consists of 4 phases.1 The first phase starts immediately after the surgical incision, with the exposure of platelets that activate the coagulation cascade. A few hours after this hemostatic phase, the inflammatory phase begins, with the recruitment initially of neutrophils and, 3 to 4 days later, of macrophages. The function of these cells is to clean the wound of foreign matter, necrotic residues, and bacteria. Under normal conditions the neutrophils are present for only a few days, but in cases of contamination their action can be prolonged. In this inflammatory phase, which usually begins 2 to 4 days later, abundant neutrophils and vessels occluded by fibrin-rich thrombi can also be observed.
The activated macrophages secrete growth factors that stimulate the formation of granulation tissue, initiating the third, proliferative phase. Fibroblasts migrate towards the wound and produce a provisional matrix of fibrin, fibronectin, and hyaluronic acid that is gradually replaced by a collagen matrix. The final, remodeling phase takes place during the following 6 to 18 months, with the appearance of contractile myofibroblasts and the production of type I collagen, which imparts tensile strength to the scar. Wound contraction is achieved through the action of actin-rich myofibroblasts and fibronectin and is proportional to the depth of the wound.
Contraction also depends on the site of the wound. In wounds in periorificial areas retraction tends to act on the mobile tissue surrounding the orifice, and second-intention closure is therefore not indicated in such cases.
In general, second-intention healing is more suited to concave surfaces than to convex ones and cosmetic results in such areas as the medial canthus of the eye, the concha of the ear, the nasolabial folds, and the preauricular and retroauricular areas may be more satisfactory.
With regard to wound depth, it should be borne in mind that, with proper care, total regeneration is possible, provided the tissue is well vascularized. The exposure of cartilage or bone delays or prevents the formation of granulation tissue and in such cases it is necessary to make several incisions from which the tissue can regenerate.
When second-intention closure is chosen, wound care should ensure a moist environment that will encourage the formation of granulation tissue.2
Petrolatum gauze (with or without antibiotics) placed over the entire surface of the surgical defect can adhere to deep wounds or those in which other tissues, such as bone or cartilage, are exposed. This makes removal of the dressing difficult and extremely painful. To avoid this complication, silicone dressings or polyurethane foams are used.
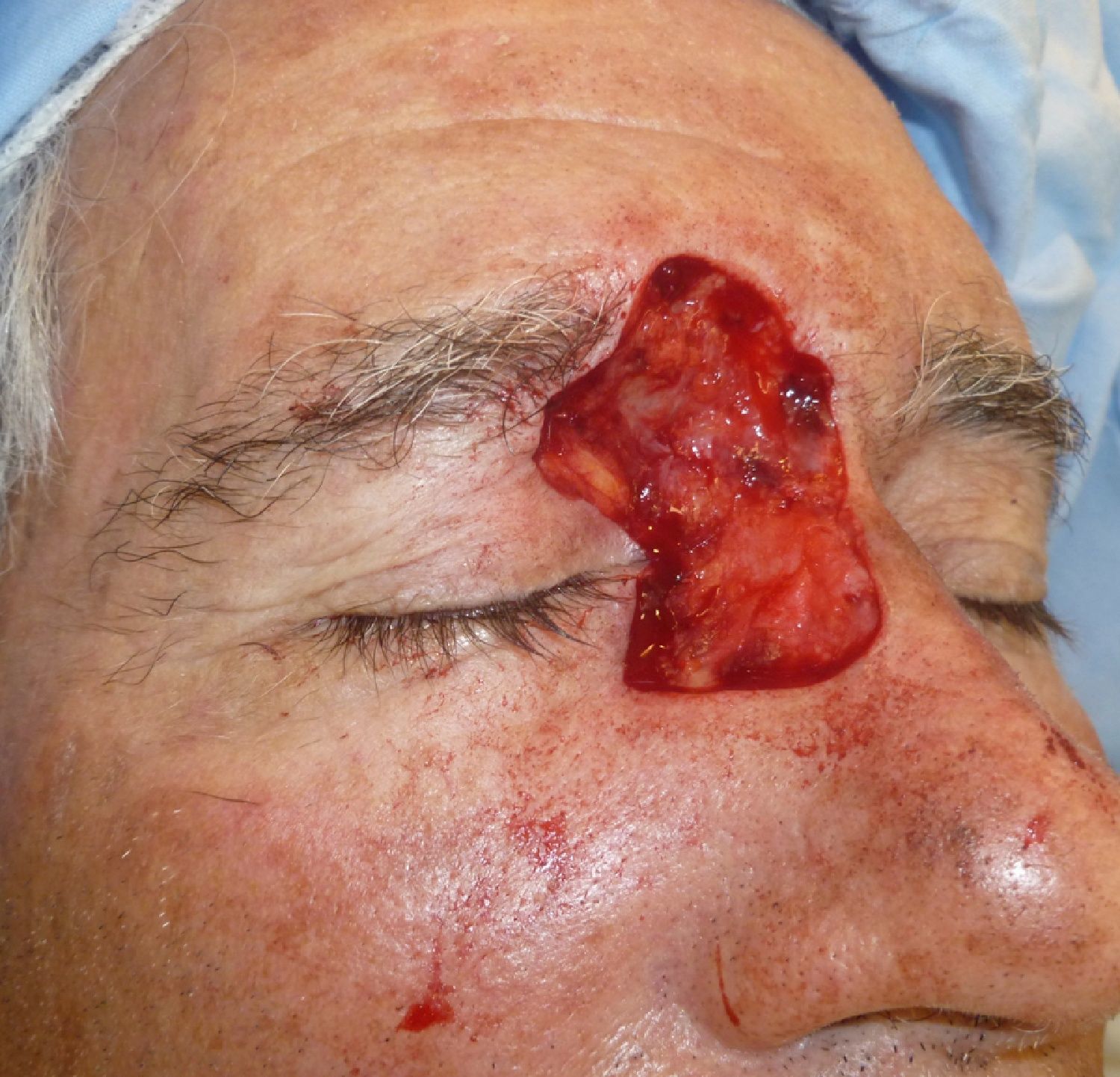
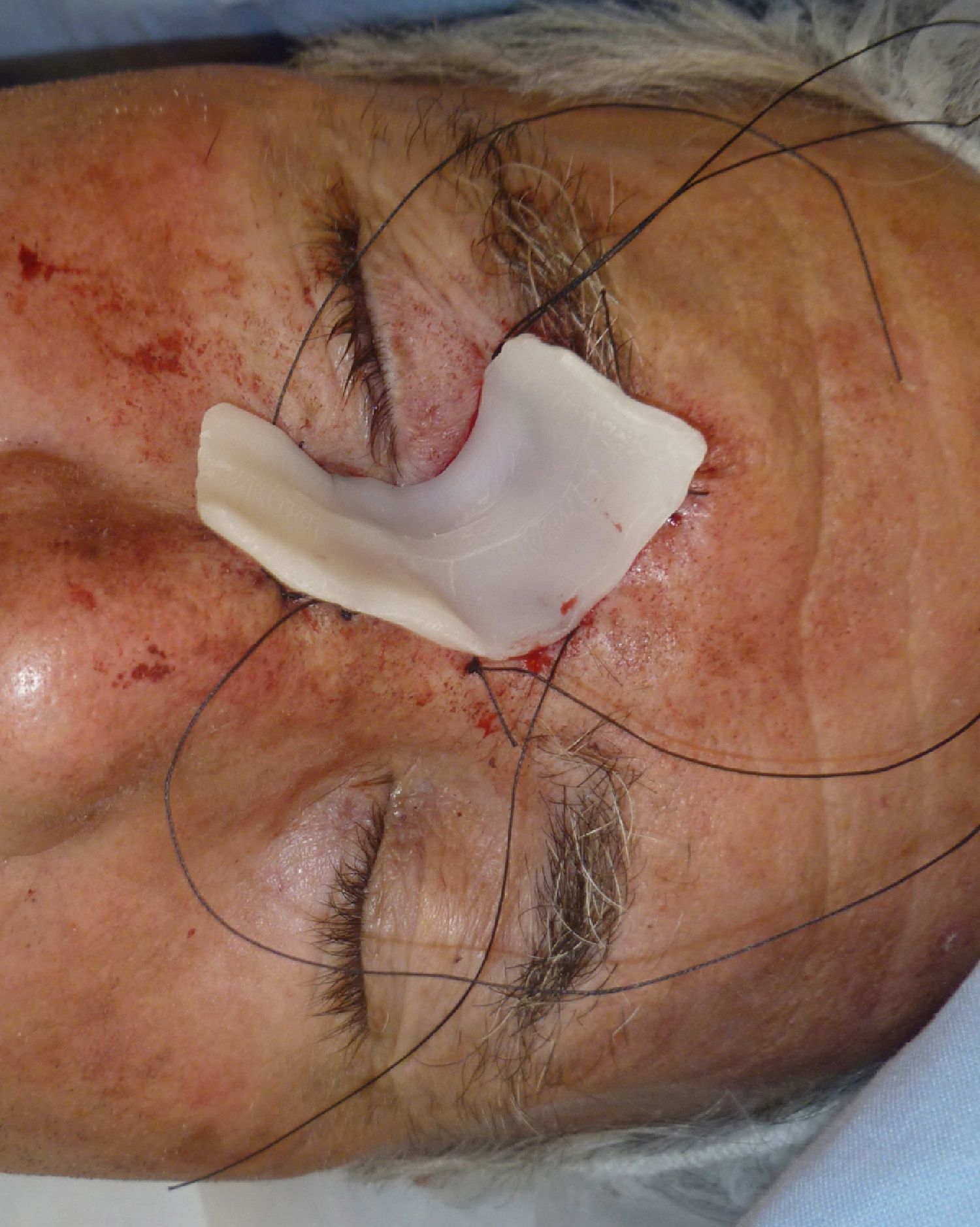
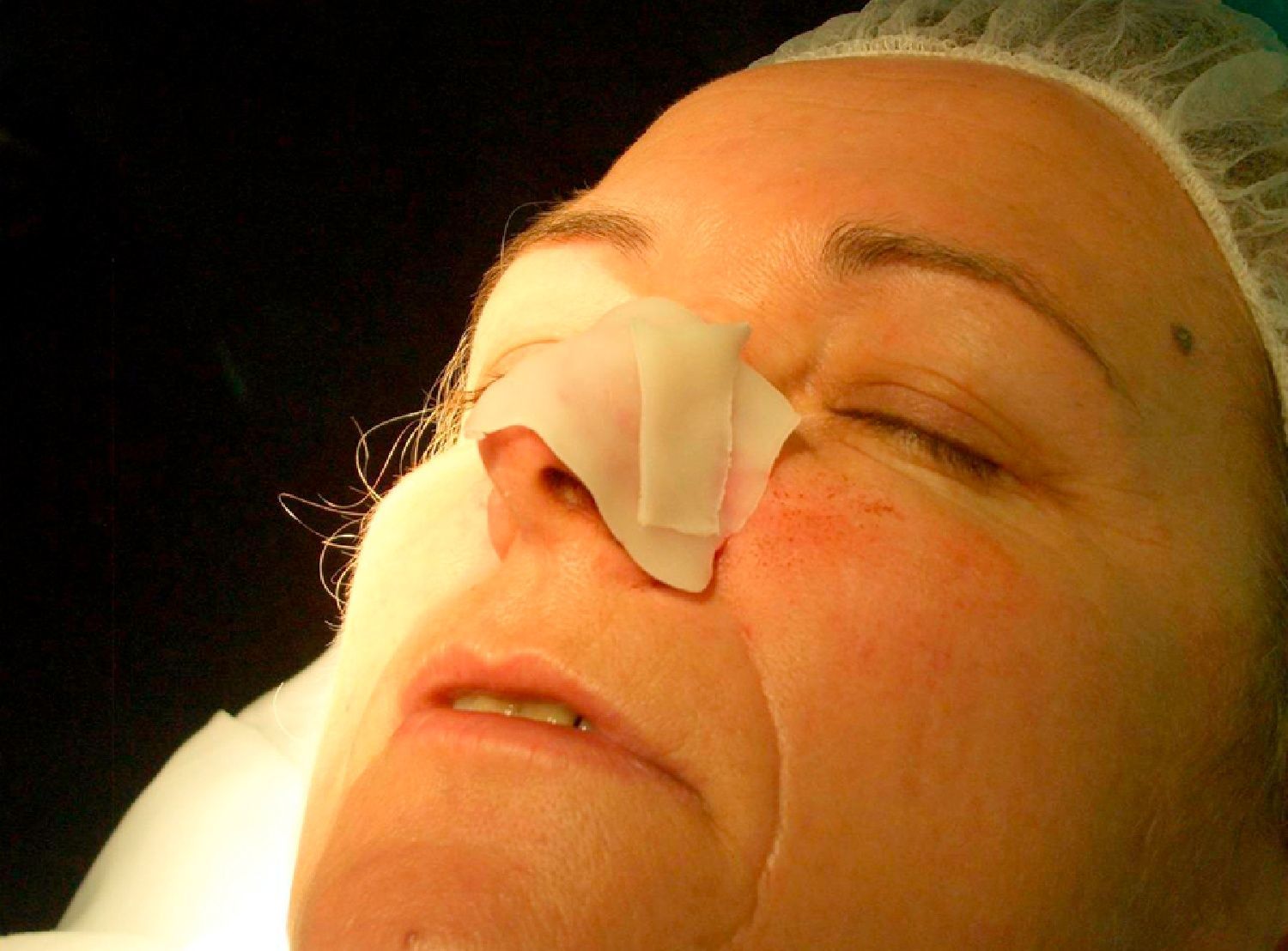
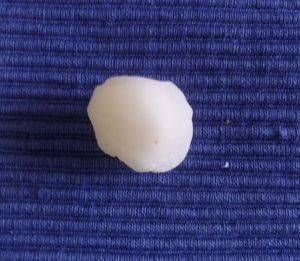
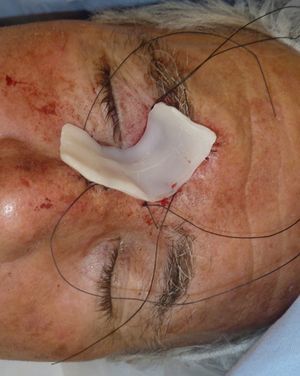
In our patients we have used bone wax, a mixture of beeswax, isopropyl palmitate, and a stabilizing agent. It was originally used for hemostasis because of its purely mechanical tamponade effect on the bleeding open channels in bone. It is a biologically inert material that does not cause skin allergy or irritant dermatitis, is not absorbed, and remains on the wound without adhering to it. This makes removal easy and painless. Furthermore, it has the great advantage of being malleable. It is supplied as a fine rectangular block (Fig. 1), but when warmed in the hands it can be molded to fit perfectly over the curvatures of the surgical defect (Fig. 2). This quality is especially advantageous in such areas as the ear, nose, or medial canthus of the eye, where some dressings might not apply pressure to the deep part of the wound or may produce tenting over the wound.
But in addition to these qualities, bone wax has added advantages for second-intention healing in dermatologic surgery; whether because of the restorative and emollient properties of the beeswax or because of the moist occlusive microenvironment it creates, good granulation tissue is observed when the bone wax is removed a week after application.
As examples, we present 2 patients in whom we used bone wax with very good results:
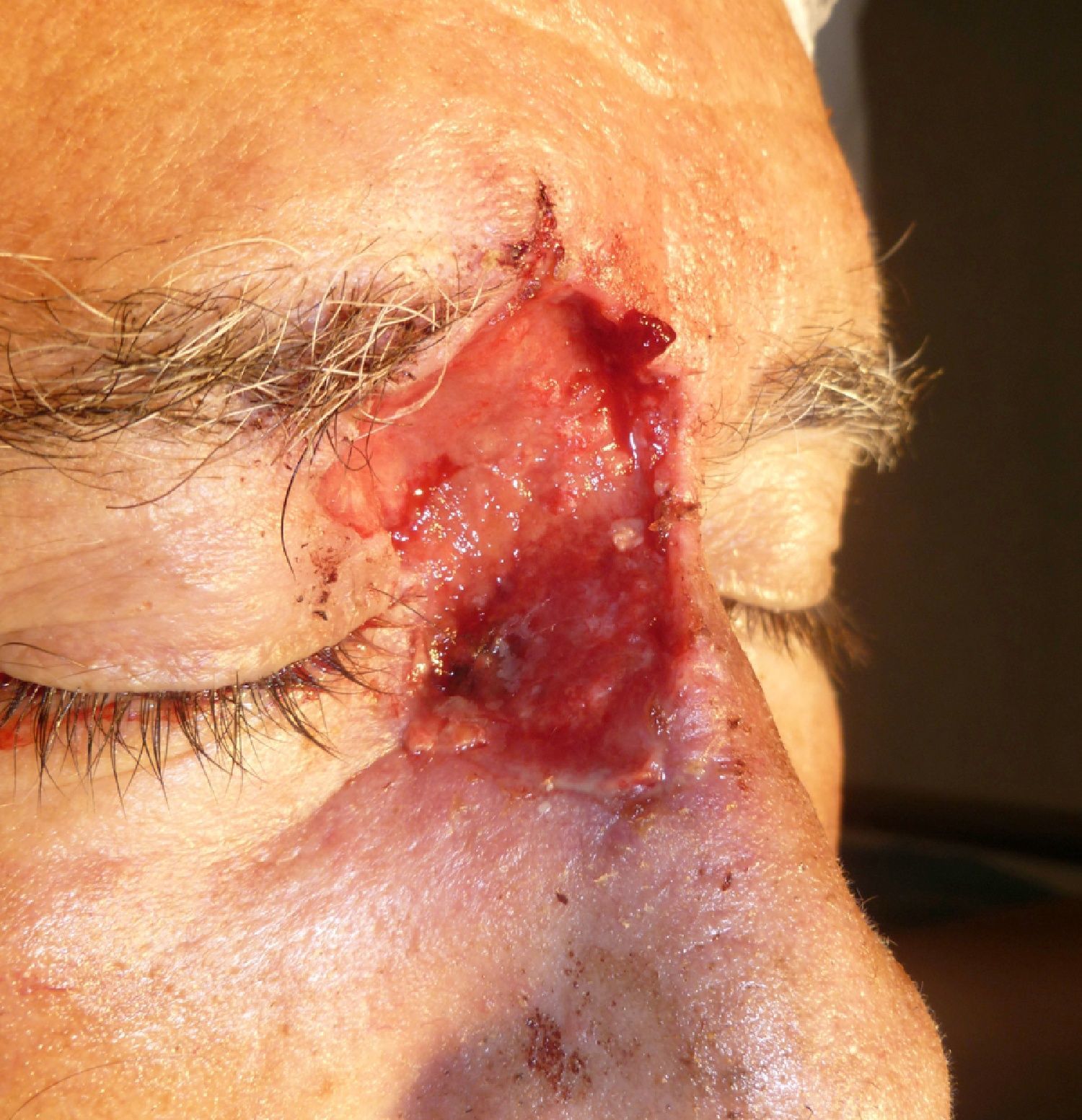
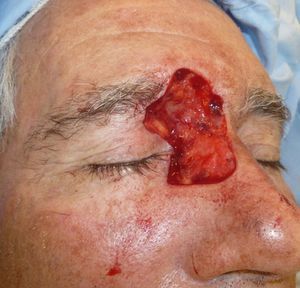
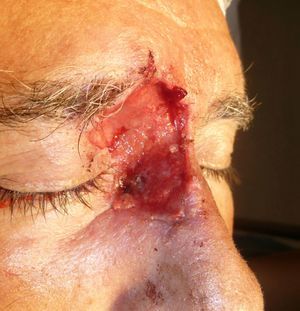
Patient 1 was a 73-year-old man with recurrent infiltrative basal cell carcinoma around the medial canthus of his right eye in whom, after the excision of the tumor, we chose to undertake reconstruction with a graft in a second surgical intervention. Figure 3 shows a deep surgical defect after 3 stages of Mohs surgery. Bone wax was applied for second-intention healing (Fig. 4). It was removed after a week and tissue regeneration was observed within the wound. The wound was not as deep and showed adequate granulation (Fig. 5).
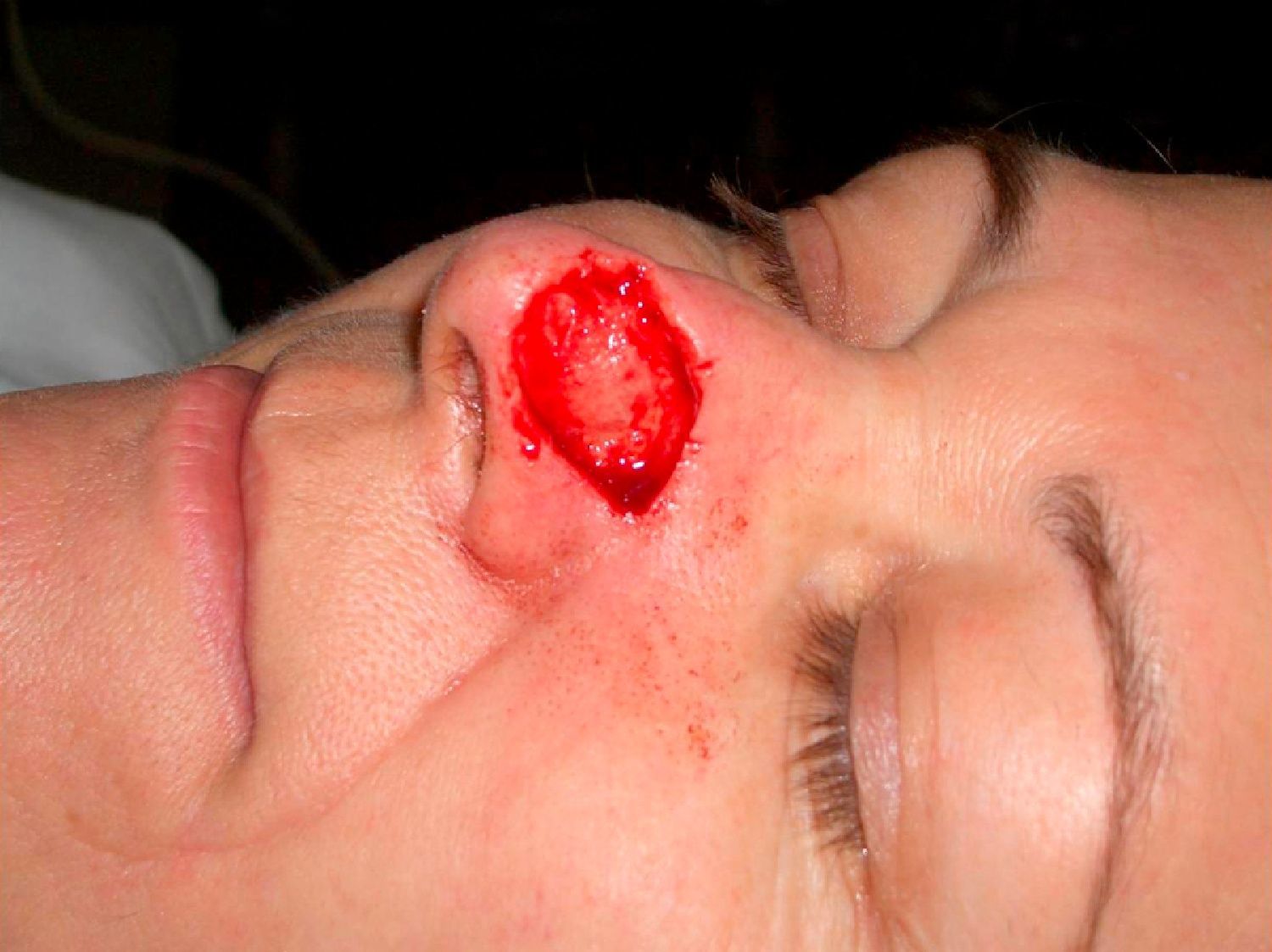
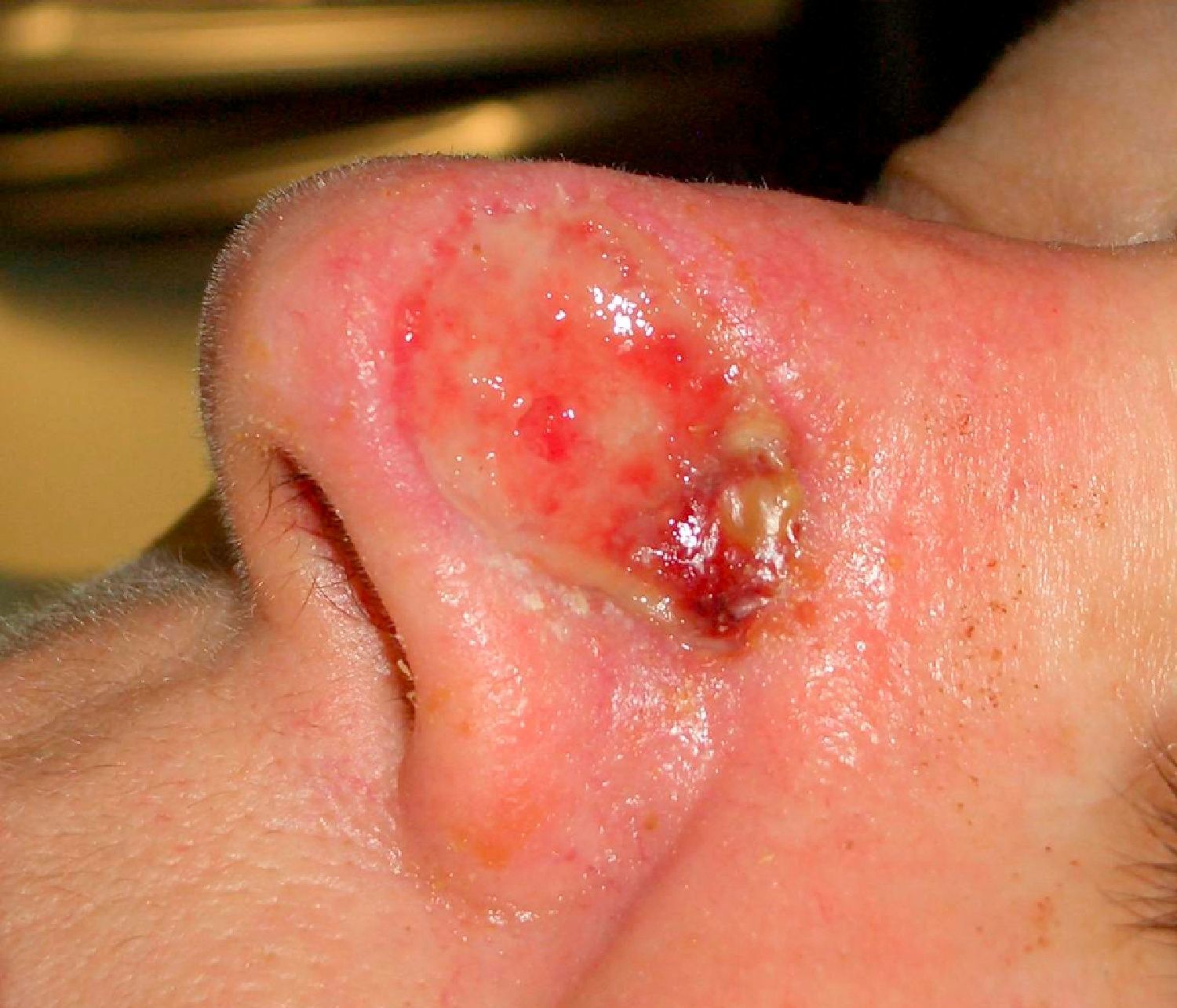
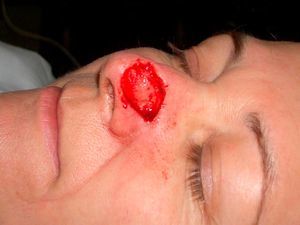
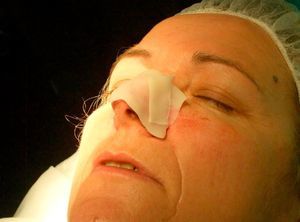
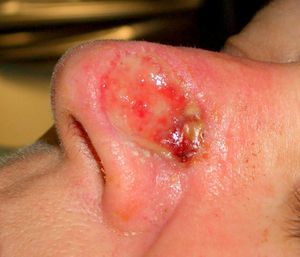
Patient 2 was a 64-year-old woman with infiltrative basal cell carcinoma on the left side of her nose who underwent Mohs surgery that resulted in a deep surgical defect, with exposure of the cartilage (Fig. 6). Bone wax was applied to cover the entire depth of the defect (Fig. 7) and was removed 1 week later. Excellent regeneration of the wound was observed (Fig. 8).
Second-intention healing is a useful therapeutic alternative in dermatologic surgery. It can be employed as the sole reconstructive option or to reduce the surgical defect, with reconstruction performed in a second operation. Its disadvantage is contraction, which is greater in periorificial areas, and it is therefore not recommended in the lower orbital region, due to the resulting ectropion, or near the lips. In hairy areas, second-intention healing gives rise to hairless scars.
It is important to evaluate the patient's general condition, as immunodepression or the presence of certain conditions such as diabetes mellitus, coagulation disorders, smoking, or alcoholism do not favor second-intention healing and increase the risk of infection or necrosis.3
Although the use of bone wax in cranial surgery was first described by Henri Ferdinand Dolbeauen4 in 1842, it was Victor Horsley who popularized its use in neurosurgery in 1892. Horsely named his preparation, composed of beeswax, salicylic acid, and almond oil, bone wax and used it to control the massive bleeding from the skulls of the dogs he used in his experiments.
Its use later spread to neurosurgery, heart surgery (for the hemostasis of sternotomies), and orthopedic surgery. It achieves hemostasis through the mechanical, rather than chemical, effect of occluding the bleeding vessels.5
However, in these types of surgery, the bone wax remains on the bleeding bone indefinitely and cases of foreign body granulomas with local inflammation and pain have been reported.6,7
Some authors have suggested, on the basis of animal experiments, that bone wax interferes with osteogenesis.8 However, the decrease in mortality associated with reduced bleeding in craniofacial surgery would justify its use despite its possible negative effect on bone formation.9
In orthodontia, bone wax is used to prevent injury to the mucosa caused by dental braces.10
In plastic surgery, bone wax has been used only as a 3-dimensional mold for nasal defects requiring a chondrocutaneous graft harvested from the ear.11 The mold can be used to choose the best site of the donor area in terms of shape and size.
In dermatologic surgery, bone wax is in some ways ideal, as it is nonadherent, deformable, and can be adapted to the surgical defect. It acts as an excellent hemostatic barrier where the skin has been excised and, although the exact mechanism is unknown, it favors tissue regeneration. Comparative studies with conventional dressings are needed in order to confirm this property.
Using it as a temporary measure avoids the risk of granulomatous complications as have been reported in bones, where it remains indefinitely.
Another advantage of bone wax over other types of dressings is its low cost (approximately €2 per unit).
In conclusion, bone wax may be a very useful therapeutic alternative for extensive and deep surgical wounds.
Ethical DisclosuresProtection of Human and Animal SubjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of DataThe authors declare that they followed their hospital's regulations regarding the publication of patient information and that written informed consent for voluntary participation was obtained for all patients.
Right to Privacy and Informed ConsentThe authors obtained informed consent from the patients and/or subjects referred to in this article. The corresponding author is in possession of this document.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Alegre M, et al. Cera para huesos en la cirugía dermatológica. Actas Dermosifiliogr. 2013;104:299–303.