The aim of this study was to analyze trends in the incidence of skin cancer worldwide, in Europe, and in Spain between 1978 and 2007.
Material and methodsSkin cancer incidence and trends for the period 1978 to 2007 were investigated using the age- and sex-standardized rates (per 100000 population) published in the Cancer Incidence in Five Continents series.
ResultsThe incidence of cutaneous melanoma increased progressively from 1978 to 2002 but decreased in the last period analyzed (2003-2007). The highest rates were reported for Australia and the white population in Hawaii. In Spain, the incidence of melanoma tripled in both sexes over the study period. The incidence of nonmelanoma skin cancer also increased between 1978 and 2007, and higher rates were detected in men. The highest incidence rates were recorded in Australia, Brazil, and among the European inhabitants of Zimbabwe. In Spain, the incidence of nonmelanoma skin cancer had doubled or tripled in both sexes by the end of the study period.
LimitationsWe were unable to analyze data for the period 2008 to 2012 due to a 5-year delay in the publication of data by the International Agency for Research on Cancer.
ConclusionsThe rise in the incidence of skin cancer, assessed using age-standardized rates, suggests that primary prevention measures are insufficient or inappropriate. The reduction in the incidence of cutaneous melanoma in Australia between 2003 and 2007 suggests that the preventive strategies initiated several decades earlier in that country have been effective.
El objetivo del estudio es analizar la tendencia temporal en la incidencia del cáncer de piel a nivel mundial, europeo y español durante el período comprendido entre 1978-2007.
Material y métodosSe estudiaron la incidencia y la tendencia del cáncer de piel en el período 1978-2007 a través de la publicación Cancer Incidence in Five Continents usando las tasas estandarizadas por edad y sexo por 100.000 habitantes.
ResultadosLa incidencia del melanoma cutáneo ha aumentado desde 1978 a 2002, pero en el último periodo 2003-2007 disminuye a nivel mundial. Las incidencias máximas se registraron en Australia y en la población de raza blanca de Hawaii. En España la incidencia de melanoma se triplicó en ambos sexos al final del período. La incidencia del cáncer cutáneo no melanoma aumentó durante el período de estudio (1978-2007), con tasas más elevadas en varones. Las incidencias máximas se registraron en Australia, Brasil y en la población europea de Zimbabue. En España la incidencia de cáncer cutáneo no melanoma llegó a duplicarse o triplicarse en ambos sexos al final del período.
LimitacionesNo se ha podido analizar el período más actual 2008-2012 debido a un retraso de 5 años en la publicación de los datos por parte del IARC.
ConclusionesEl aumento de la incidencia del cáncer de piel ajustando por los cambios en el envejecimiento de la población sugiere que las medidas de prevención primaria son insuficientes o inadecuadas. La disminución de la incidencia de melanoma en Australia en el último período apoya la eficacia de medidas de prevención iniciadas hace varias décadas.
Melanoma and nonmelanoma skin cancer (NMSC) constitute a global health problem. Their incidence has risen in recent decades, although mortality rates have remained stable and in some cases even decreased.1–8
Lip cancer is recorded as a separate entity in cancer registries, and in the Cancer Incidence in Five Continents series it is registered under a different code to that used for NMSC. However, most registered cases of lip cancer are squamous cell carcinoma (SCC).
Exposure to UV radiation, whether from the sun or artificial sources, is the greatest environmental risk factor for skin cancer. UV radiation is a known carcinogen and excessive exposure increases the risk of skin and lip cancer, particularly in individuals with low Fitzpatrick skin types.
The association between sun exposure patterns and cancer risk varies and is supported by different levels of evidence. Cutaneous melanoma and BCC, for example, have been linked to intermittent or acute sun exposure (sunburn before the age of 20 years), while SCC has been linked to chronic, cumulative exposure.3,8,9
The risk of developing cancer is even greater when exposure to UV radiation is combined with other risk factors, such as genetic predisposition, low Fitzpatrick skin type, a history of sunburn in childhood and adolescence, human papilloma virus infection, multiple dysplastic nevi, actinic keratosis, and immunosuppressed states.10–13
Cutaneous melanoma affects a higher proportion of young patients (mean age at diagnosis, 57 years). It is also more common in younger women, although the number of new cases in men increases significantly after the age of 55 years.14,15 It is also considered to be one of worst cancers in terms of potential years of life lost, premature death, and associated morbidity.16,17
NMSC is the most common skin cancer, and while mortality is low, it constitutes a significant economic burden for the health care system.18 NMSC incidence increases with age and is greater in men than in women. The fact that the risk of SCC and BCC is associated with chronic and acute exposure to UV radiation, respectively, makes NMSC susceptible to targeted primary prevention measures19 promoting healthy sun habits, such as avoidance of sunburn in childhood and limitation of exposure to UV radiation.
Population-based cancer registries provide insights into the magnitude of incidence and mortality of different types of skin cancer in the general population, and they also show temporal trends and facilitate comparisons between populations.20 The aim of this study was to analyze temporal trends in the incidence of skin cancer (cutaneous melanoma, NMSC, and lip cancer) over a recent period of 30 years based on data published by the International Agency of Research on Cancer (IARC).
Material and MethodsWe reviewed incidence rates for cutaneous melanoma, NMSC, and lip cancer reported in the Cancer Incidence in Five Continents series published by the IARC within the framework of the World Health Organization. We analyzed the 6 volumes available to date: V (1978-1982),21 VI (1983-1987),22 VII (1988-1992),23 VIII (1993-1997),24 IX (1998-2002),25 and X (2003-2007).26
These volumes record age- and sex-standardized incidence rates for different types of cancers, categorized by site, in populations around the world. The reference population is the world standard population. Each volume reports data for a 5-year period but there is a delay of 5 years between the period studied and the publication of the results due to the difficulty of gathering and exhaustively analyzing the large volumes of data involved. The latest period analyzed in our study was the period 2003 to 2007, which corresponds to the most recent volume, published in 2014.26
We analyzed temporal trends in the incidence of NMSC, cutaneous melanoma, and lip cancer among males and females worldwide, in Europe, and in Spain between 1978 and 2007 using age-standardized rates (ASRs) per 100000 population based on the world standard population and compared maximum incidence rates registered for the different 5-year periods. We also compared the incidence of NMSC, cutaneous melanoma, and skin cancer between males and females and performed a more detailed analysis of Spanish registries.
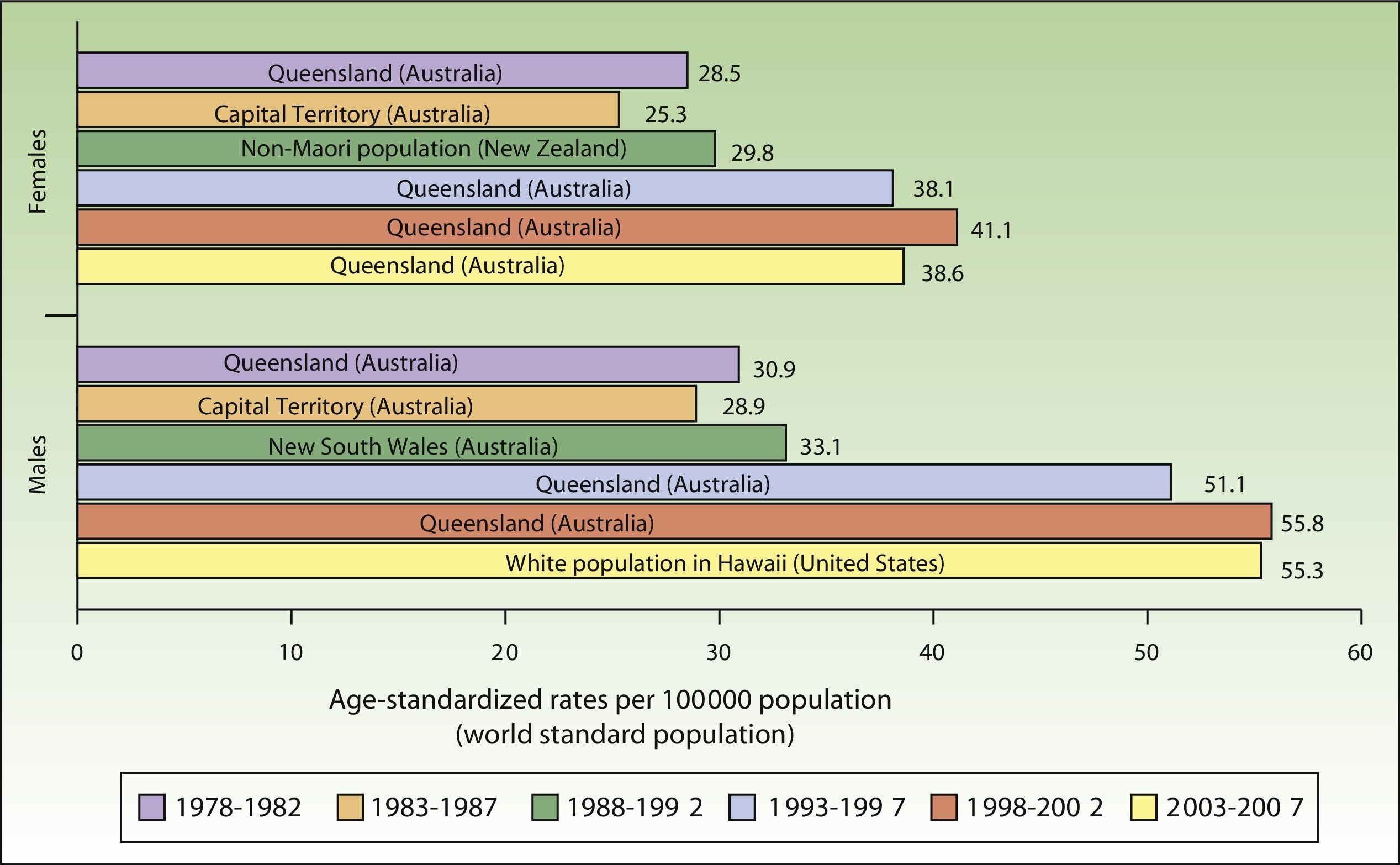
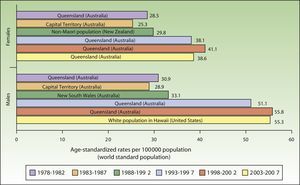
ResultsIncidence and Temporal Trends for Cutaneous Melanoma Between 1978 and 2007Incidence rates for cutaneous melanoma increased over the study period up to the last period (2003-2007), when a decline was observed for both males and females. The highest rates for both sexes were observed in Queensland, Australia for the period 1998 to 2002, with an ASR of 41.1 cases per 100000 females and 55.8 cases per 100000 males. The maximum number of new cases was higher in males. The highest incidence rates observed for melanoma for the 30-year period were in Australia (Fig. 1).
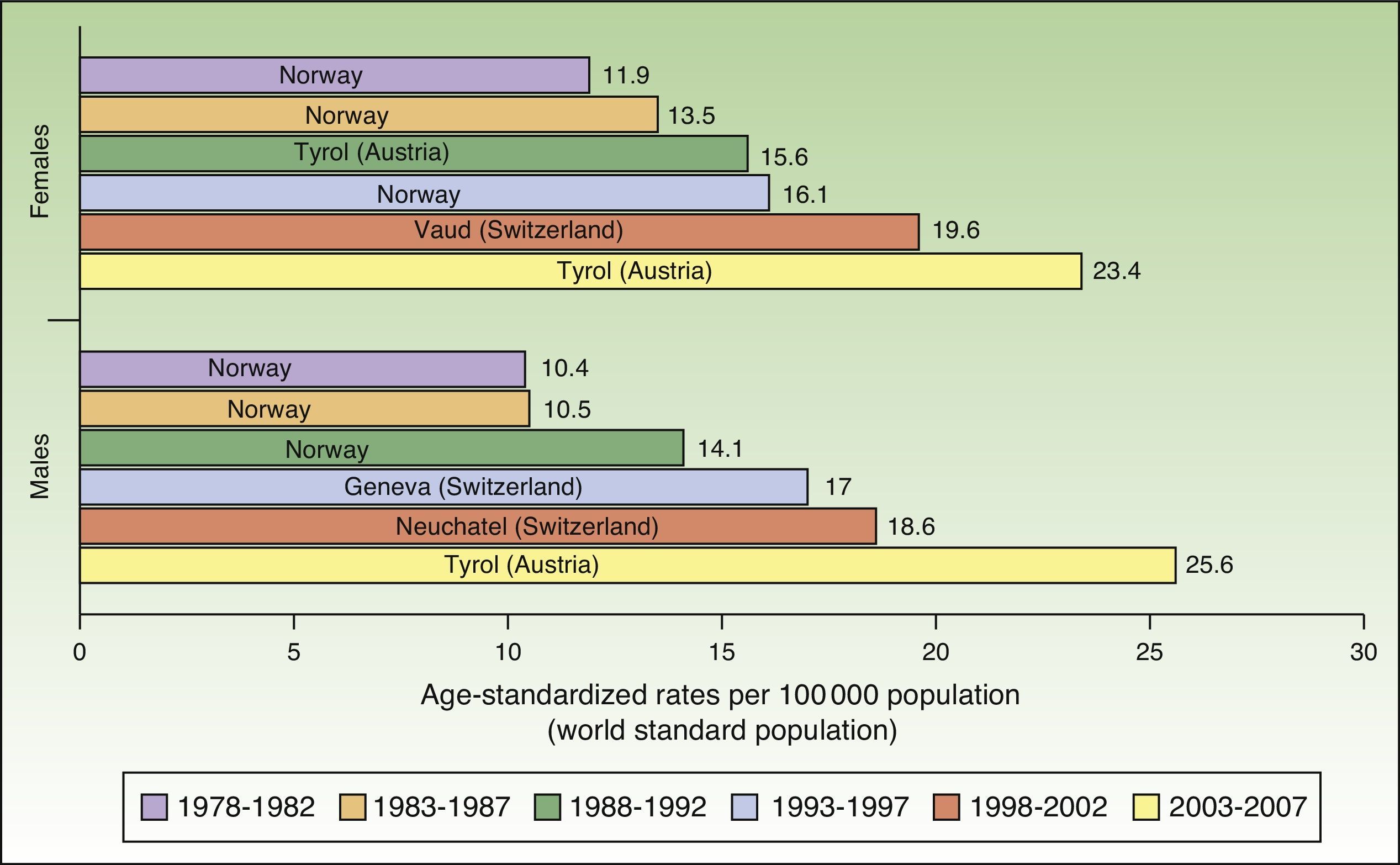
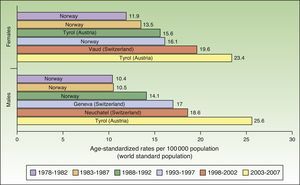
The number of new cases of melanoma in Europe also increased progressively between 1978 and 2007. The highest rates for both sexes were observed in Tyrol, Austria between 2003 and 2007, with an ASR of 23.4 cases per 100000 females and 25.6 cases per 100000 males. Maximum rates were consistently higher in females during the first 5 periods, but men took over in the last period (2003-2007). Norway and Switzerland were the top-ranking European countries for melanoma incidence (Fig. 2).
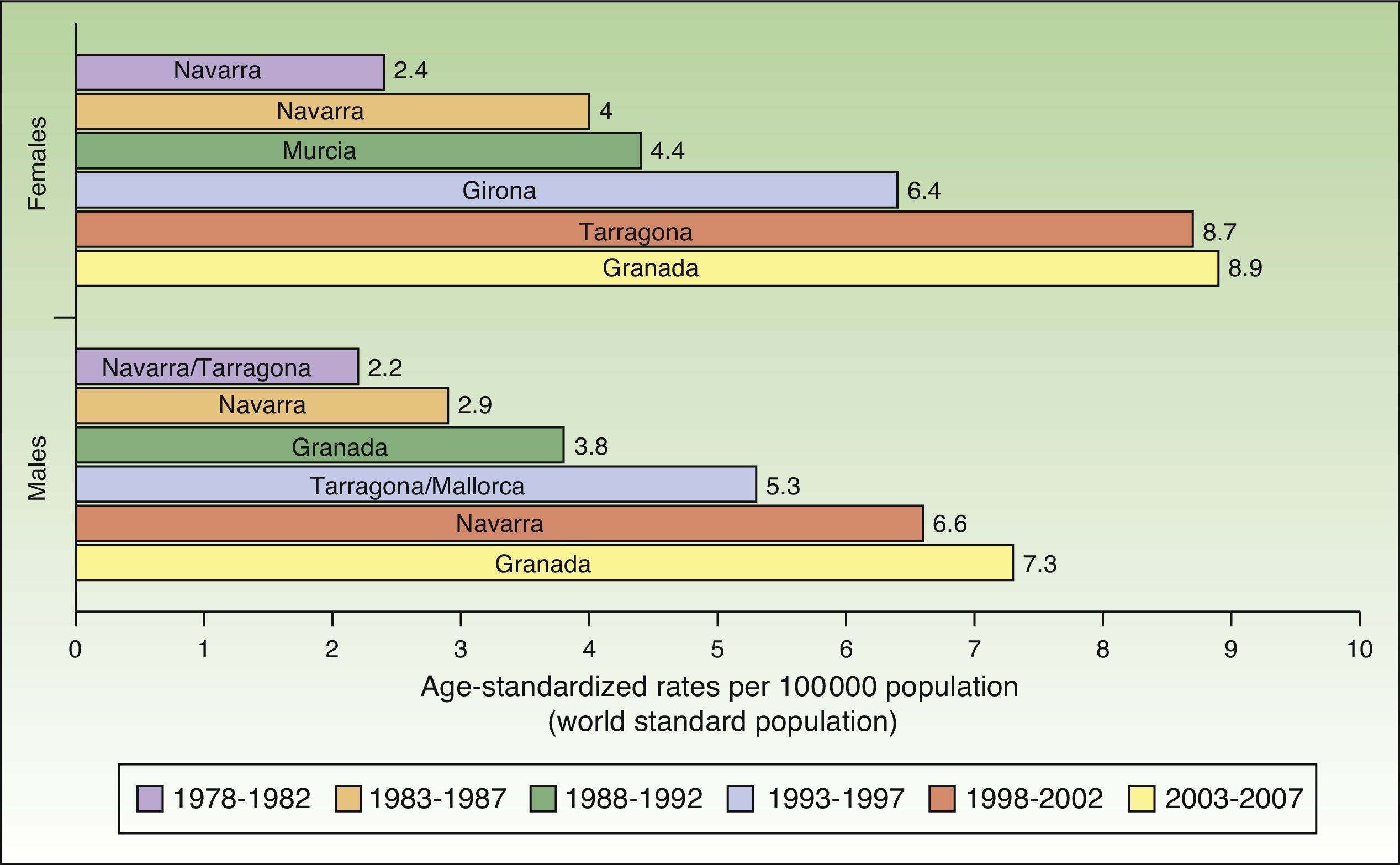
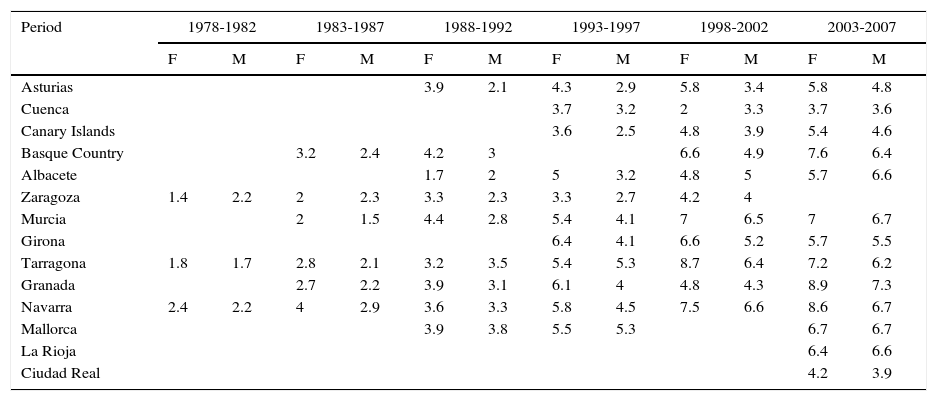
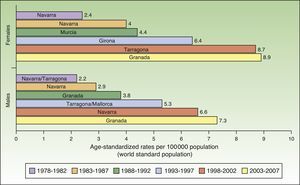
The incidence of melanoma in Spain also increased over the 30-year period analyzed. The highest rates for both sexes were recorded in Granada, Spain in the last period (2003-2007), with an ASR of 8.9 cases per 100000 females and 7.3 cases per 100000 males. Females had consistently higher rates than men. The highest melanoma incidence rates were in Mallorca, Tarragona, Navarra, Murcia, Girona, and Granada (Table 1, Fig. 3).
Incidence of Cutaneous Melanoma in Females (F) and Males (M) in Spanish Cancer Registries Between 1978 and 2007.
| Period | 1978-1982 | 1983-1987 | 1988-1992 | 1993-1997 | 1998-2002 | 2003-2007 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | M | F | M | F | M | F | M | F | M | F | M | |
| Asturias | 3.9 | 2.1 | 4.3 | 2.9 | 5.8 | 3.4 | 5.8 | 4.8 | ||||
| Cuenca | 3.7 | 3.2 | 2 | 3.3 | 3.7 | 3.6 | ||||||
| Canary Islands | 3.6 | 2.5 | 4.8 | 3.9 | 5.4 | 4.6 | ||||||
| Basque Country | 3.2 | 2.4 | 4.2 | 3 | 6.6 | 4.9 | 7.6 | 6.4 | ||||
| Albacete | 1.7 | 2 | 5 | 3.2 | 4.8 | 5 | 5.7 | 6.6 | ||||
| Zaragoza | 1.4 | 2.2 | 2 | 2.3 | 3.3 | 2.3 | 3.3 | 2.7 | 4.2 | 4 | ||
| Murcia | 2 | 1.5 | 4.4 | 2.8 | 5.4 | 4.1 | 7 | 6.5 | 7 | 6.7 | ||
| Girona | 6.4 | 4.1 | 6.6 | 5.2 | 5.7 | 5.5 | ||||||
| Tarragona | 1.8 | 1.7 | 2.8 | 2.1 | 3.2 | 3.5 | 5.4 | 5.3 | 8.7 | 6.4 | 7.2 | 6.2 |
| Granada | 2.7 | 2.2 | 3.9 | 3.1 | 6.1 | 4 | 4.8 | 4.3 | 8.9 | 7.3 | ||
| Navarra | 2.4 | 2.2 | 4 | 2.9 | 3.6 | 3.3 | 5.8 | 4.5 | 7.5 | 6.6 | 8.6 | 6.7 |
| Mallorca | 3.9 | 3.8 | 5.5 | 5.3 | 6.7 | 6.7 | ||||||
| La Rioja | 6.4 | 6.6 | ||||||||||
| Ciudad Real | 4.2 | 3.9 | ||||||||||
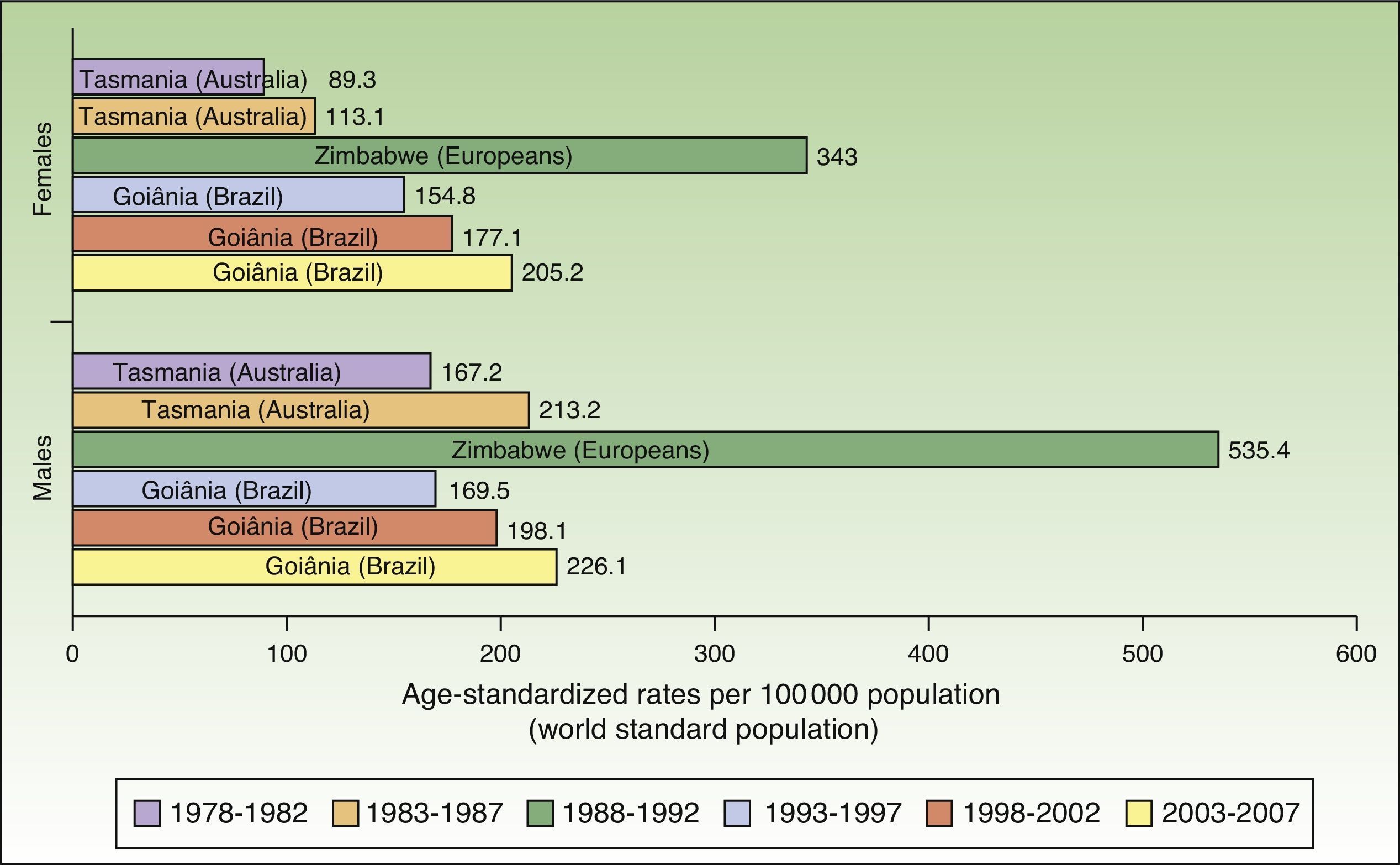
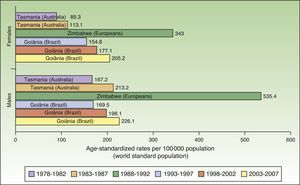
The world incidence of NMSC also increased in males and females over the study period, although a greater increase in new cases was noted in the female population. The highest rates for males and females were observed in Europeans living in Zimbabwe for the period 1988 to 1992, with a respective ASR of 535.4 and 343 cases per 100000 population. Males had the highest number of new cases. Worldwide, Australia and Brazil were the countries with the most new cases of NMSC (Fig. 4).
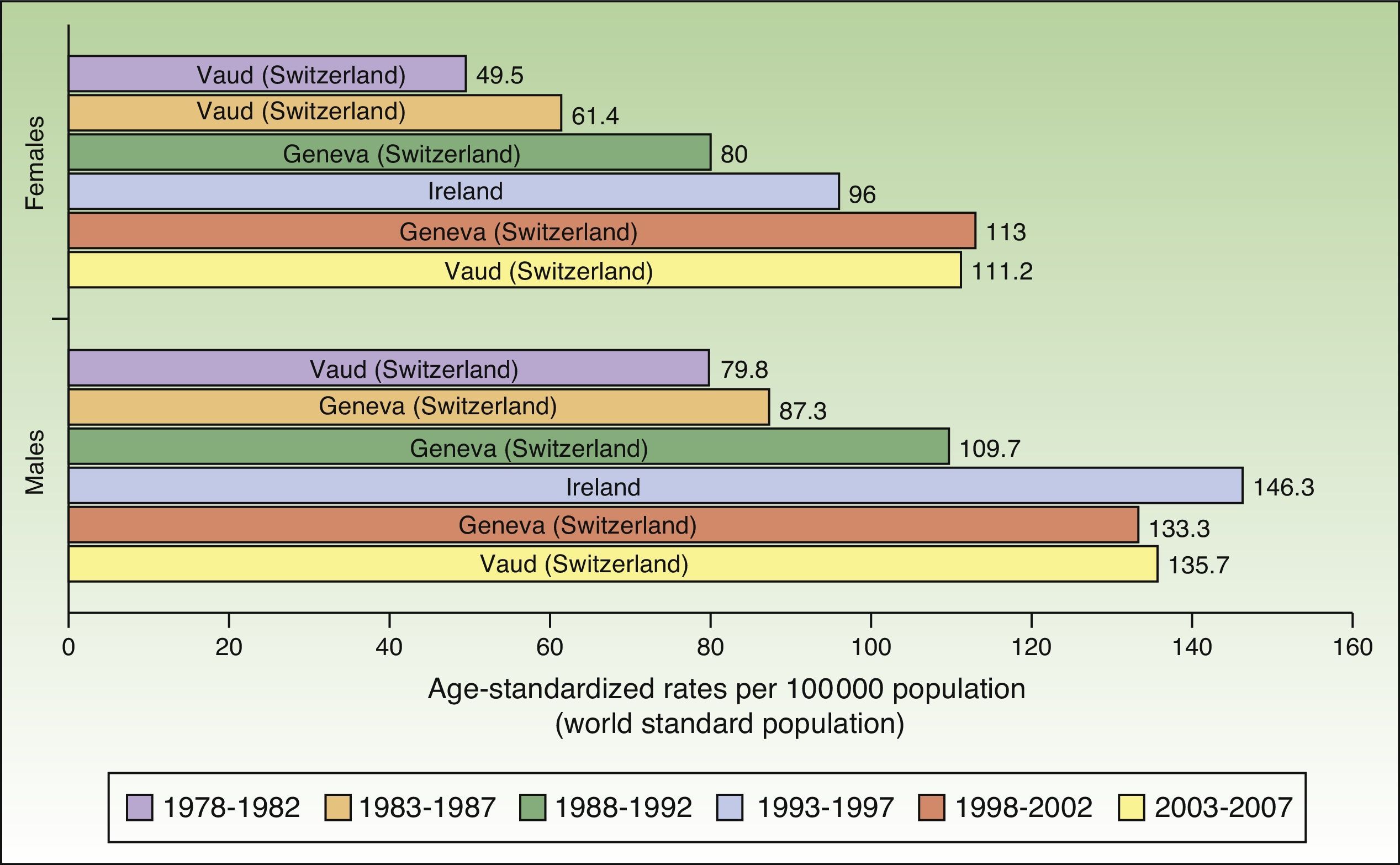
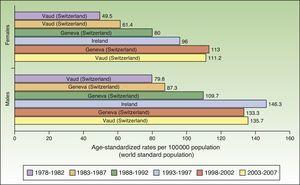
In Europe, NMSC incidence rates increased over the entire study period in males. In females, they increased up to the last period (2003-2007), when a slight decline was observed. Nevertheless, the highest rate in these last 5 years was twice as high as rates prior to the 1998 to 2002 period. Females in Geneva, Switzerland had the highest number of new cases (113 cases per 100000 population). This peak corresponded to the period 1998 to 2002. The highest rate recorded for males was in Ireland, in 1993 to 1997, with an ASR of 146.3 cases per 100000 population. Maximum rates were higher in males. Ireland and Switzerland had the highest rates of NMSC in Europe (Fig. 5).
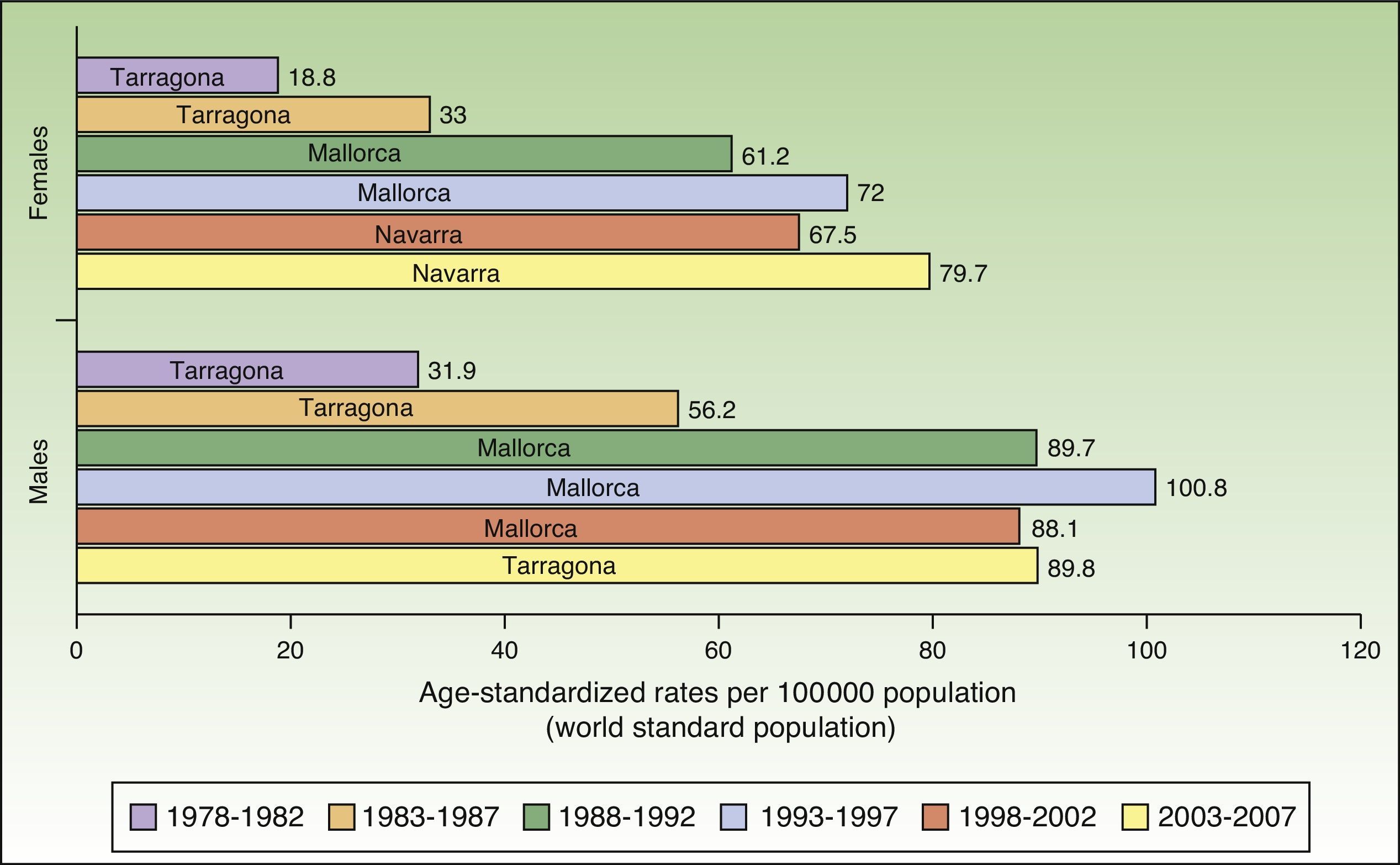
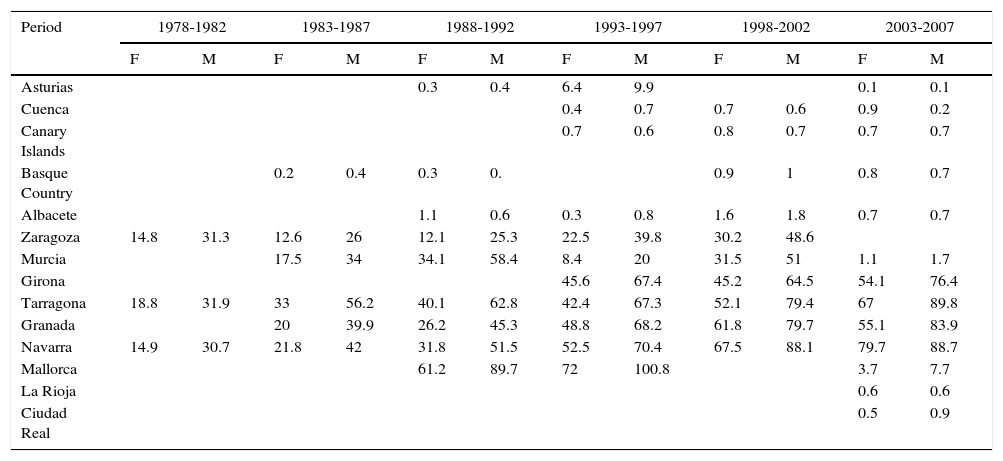
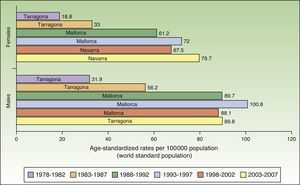
In Spain, the maximum incidence of NMSC increased considerably over the 30-year study period, with a tripling of rates in the female population. The highest rate for females was recorded in Navarra, between 2003 and 2007, with an ASR of 79.7 cases per 100000 population. For men, the highest rate was observed in Mallorca between 1993 and 1997, with an ASR of 100.8 cases per 100000 population. Maximum rates were higher in males. The Spanish regions with the highest number of new cases of NMSC were Mallorca, Tarragona, and Navarra (Table 2, Fig. 6).
Incidence of Nonmelanoma Skin Cancer in Females (F) and Males (M) in Spanish Cancer Registries Between 1978 and 2007.
| Period | 1978-1982 | 1983-1987 | 1988-1992 | 1993-1997 | 1998-2002 | 2003-2007 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | M | F | M | F | M | F | M | F | M | F | M | |
| Asturias | 0.3 | 0.4 | 6.4 | 9.9 | 0.1 | 0.1 | ||||||
| Cuenca | 0.4 | 0.7 | 0.7 | 0.6 | 0.9 | 0.2 | ||||||
| Canary Islands | 0.7 | 0.6 | 0.8 | 0.7 | 0.7 | 0.7 | ||||||
| Basque Country | 0.2 | 0.4 | 0.3 | 0. | 0.9 | 1 | 0.8 | 0.7 | ||||
| Albacete | 1.1 | 0.6 | 0.3 | 0.8 | 1.6 | 1.8 | 0.7 | 0.7 | ||||
| Zaragoza | 14.8 | 31.3 | 12.6 | 26 | 12.1 | 25.3 | 22.5 | 39.8 | 30.2 | 48.6 | ||
| Murcia | 17.5 | 34 | 34.1 | 58.4 | 8.4 | 20 | 31.5 | 51 | 1.1 | 1.7 | ||
| Girona | 45.6 | 67.4 | 45.2 | 64.5 | 54.1 | 76.4 | ||||||
| Tarragona | 18.8 | 31.9 | 33 | 56.2 | 40.1 | 62.8 | 42.4 | 67.3 | 52.1 | 79.4 | 67 | 89.8 |
| Granada | 20 | 39.9 | 26.2 | 45.3 | 48.8 | 68.2 | 61.8 | 79.7 | 55.1 | 83.9 | ||
| Navarra | 14.9 | 30.7 | 21.8 | 42 | 31.8 | 51.5 | 52.5 | 70.4 | 67.5 | 88.1 | 79.7 | 88.7 |
| Mallorca | 61.2 | 89.7 | 72 | 100.8 | 3.7 | 7.7 | ||||||
| La Rioja | 0.6 | 0.6 | ||||||||||
| Ciudad Real | 0.5 | 0.9 | ||||||||||
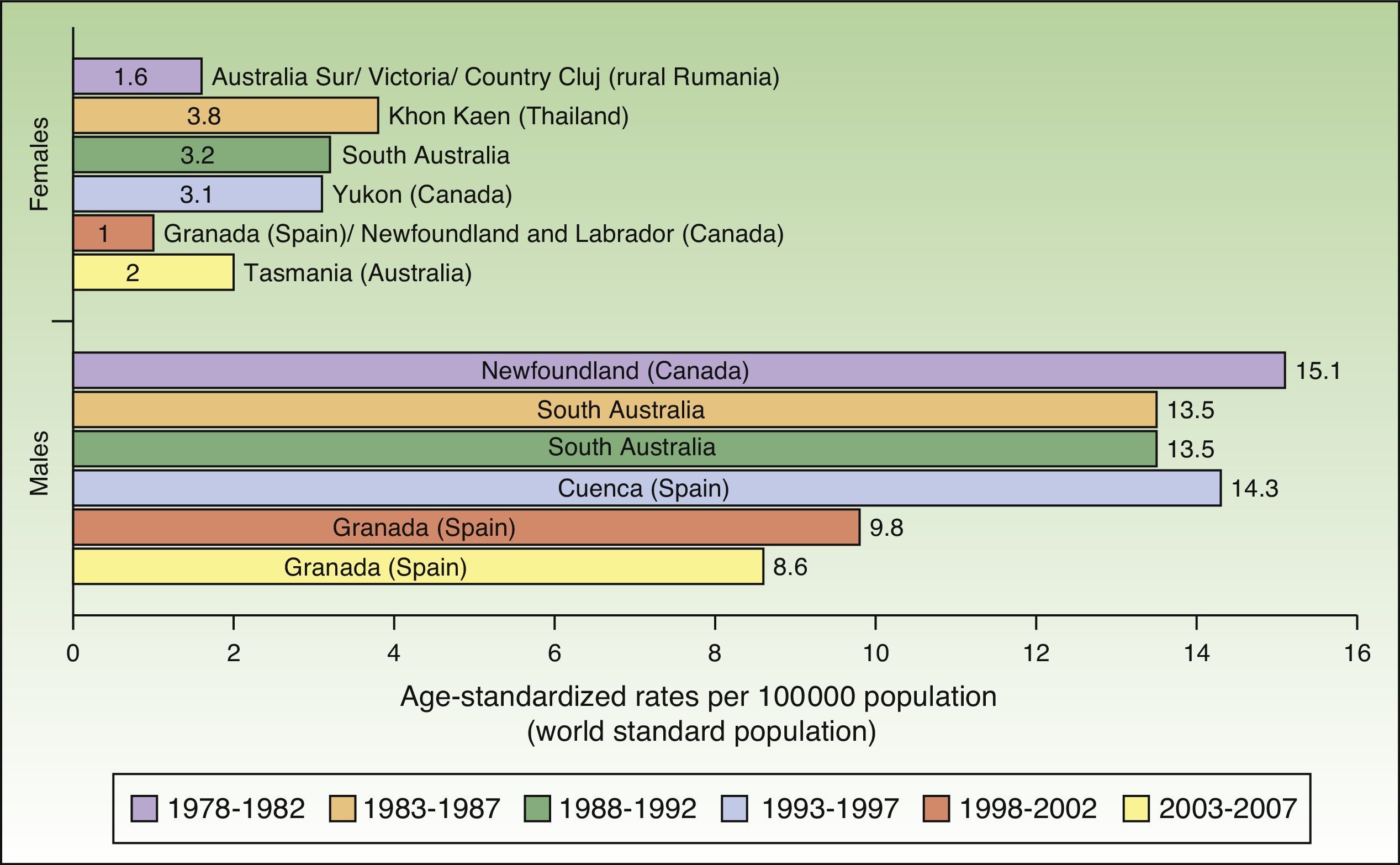
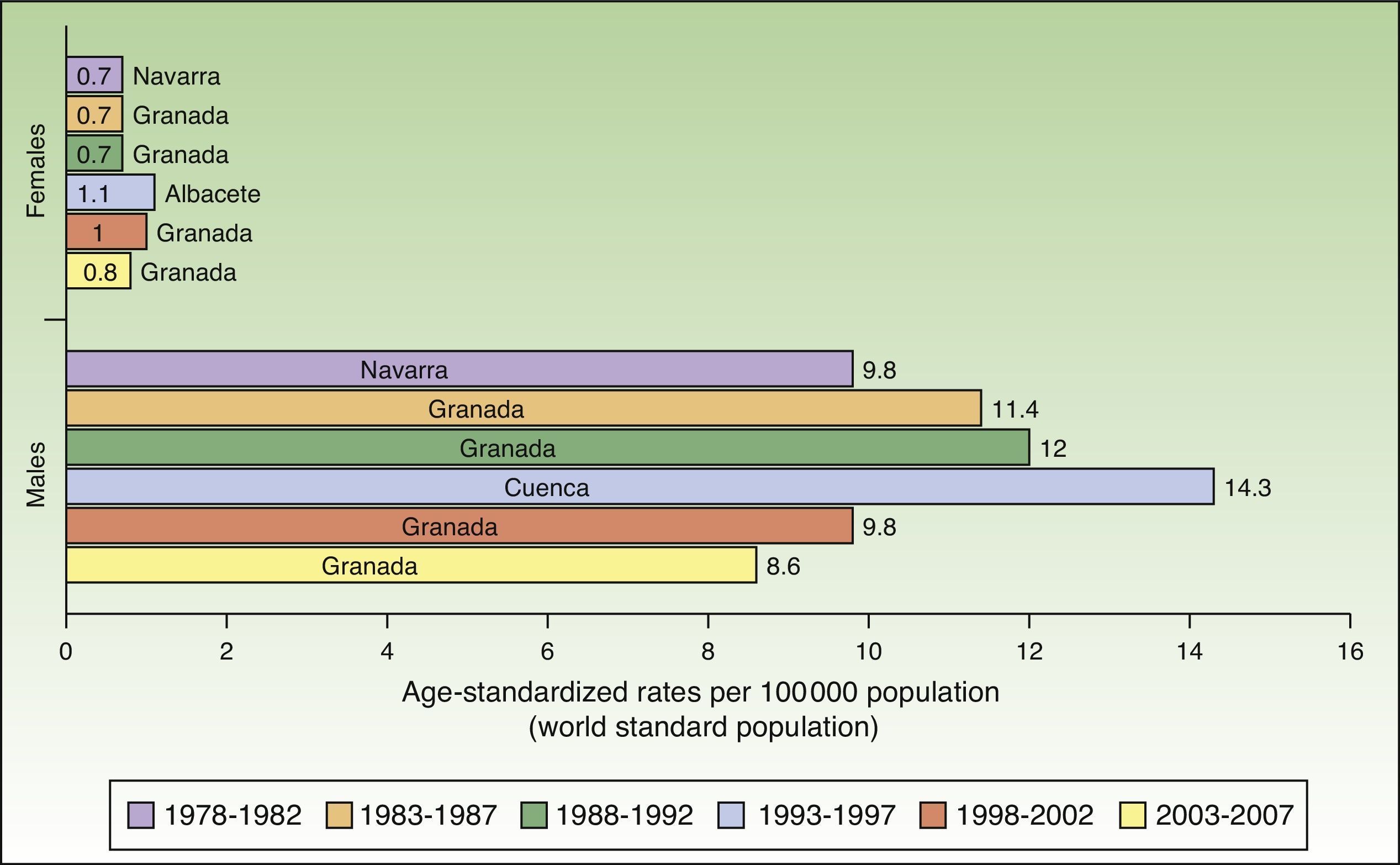
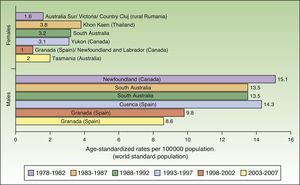
Incidence rates for lip cancer among males decreased between 1978 and 2007, and the decline was more evident in the last 2 periods. Rates in the female population tended to stabilize. The highest rate recorded for females was in Khon Kaen, Thailand during the period 1983 to 1987, with an ASR of 3.8 cases per 100000 inhabitants. The highest rate for males, by contrast, was in Newfoundland, Canada during the previous period (1978-1982), with an ASR of 15.1 cases per 100000 inhabitants. Worldwide, the highest numbers of new lip cancer cases were registered in Australia, Canada, and Spain (Fig. 7).
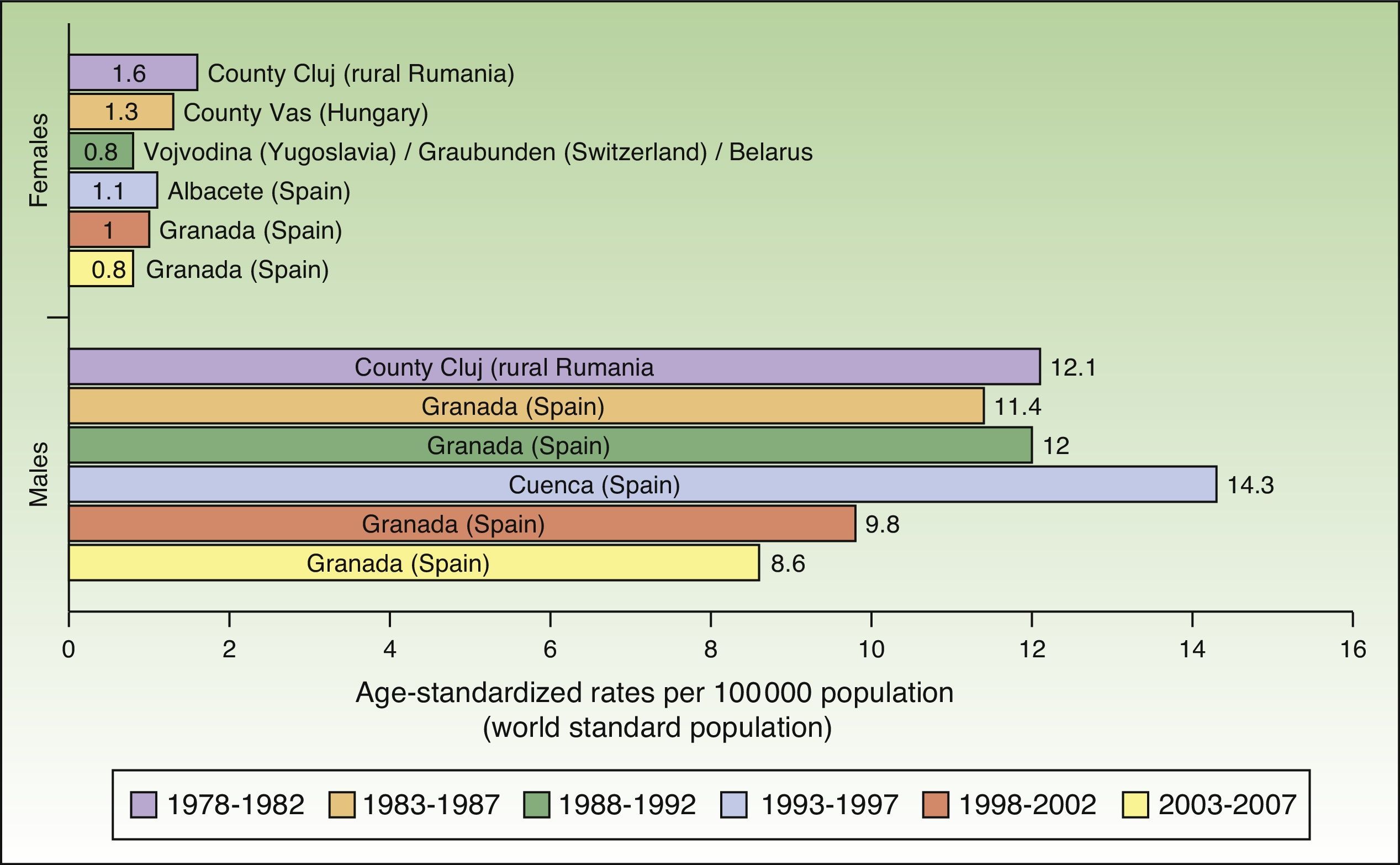
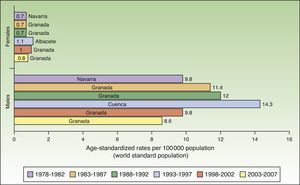
Maximum rates of lip cancer incidence also decreased in Spain over the 30-year period. The decline was more evident in males, especially during the last 10 years (1998-2007). The highest rate recorded for men was in Cuenca, Spain for the period 1993 to 1997, with an ASR of 14.3 cases per 100000 population. In women, the highest rate (1.6 cases per 100000 population) was observed in the rural population of Cluj in Romania during the period 1978 to 1982. Maximum incidence was higher among males. Spain had the highest incidence of lip cancer in Europe (Fig. 8).
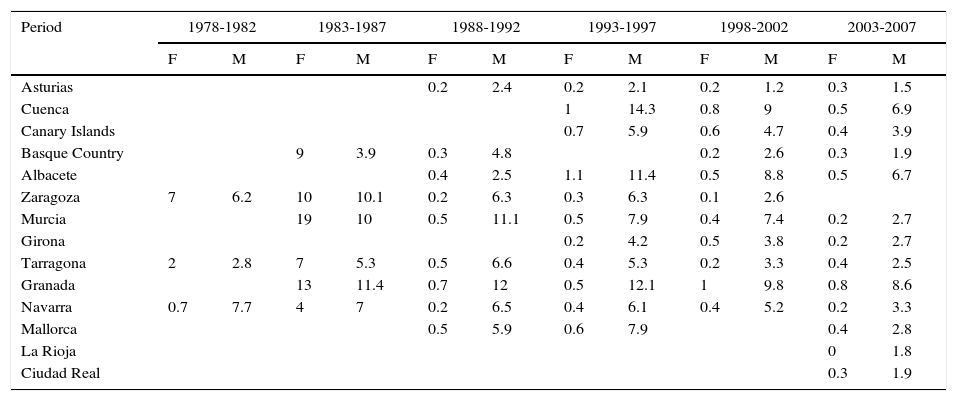
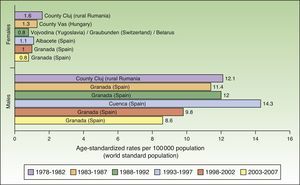
In Spain, like in Europe and the rest of the world, lip cancer rates also declined between 1978 and 2007, with a tendency towards stabilization in the female population. The highest rates were recorded in the period 1993 to 1997. In females, the highest ASR was 1.1 cases per 100000 population, registered in Albacete, while in males, it was 14.3 cases per 100000 population, registered in Cuenca. Incidence rates were highest among males. The regions with the highest rates of lip cancer in Spain were Granada, Navarra, Cuenca, and Albacete (Table 3, Fig. 9).
Incidence of Lip Cancer in Females (F) and Males (M) in Spanish Cancer Registries Between 1978 and 2007.
| Period | 1978-1982 | 1983-1987 | 1988-1992 | 1993-1997 | 1998-2002 | 2003-2007 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | M | F | M | F | M | F | M | F | M | F | M | |
| Asturias | 0.2 | 2.4 | 0.2 | 2.1 | 0.2 | 1.2 | 0.3 | 1.5 | ||||
| Cuenca | 1 | 14.3 | 0.8 | 9 | 0.5 | 6.9 | ||||||
| Canary Islands | 0.7 | 5.9 | 0.6 | 4.7 | 0.4 | 3.9 | ||||||
| Basque Country | 9 | 3.9 | 0.3 | 4.8 | 0.2 | 2.6 | 0.3 | 1.9 | ||||
| Albacete | 0.4 | 2.5 | 1.1 | 11.4 | 0.5 | 8.8 | 0.5 | 6.7 | ||||
| Zaragoza | 7 | 6.2 | 10 | 10.1 | 0.2 | 6.3 | 0.3 | 6.3 | 0.1 | 2.6 | ||
| Murcia | 19 | 10 | 0.5 | 11.1 | 0.5 | 7.9 | 0.4 | 7.4 | 0.2 | 2.7 | ||
| Girona | 0.2 | 4.2 | 0.5 | 3.8 | 0.2 | 2.7 | ||||||
| Tarragona | 2 | 2.8 | 7 | 5.3 | 0.5 | 6.6 | 0.4 | 5.3 | 0.2 | 3.3 | 0.4 | 2.5 |
| Granada | 13 | 11.4 | 0.7 | 12 | 0.5 | 12.1 | 1 | 9.8 | 0.8 | 8.6 | ||
| Navarra | 0.7 | 7.7 | 4 | 7 | 0.2 | 6.5 | 0.4 | 6.1 | 0.4 | 5.2 | 0.2 | 3.3 |
| Mallorca | 0.5 | 5.9 | 0.6 | 7.9 | 0.4 | 2.8 | ||||||
| La Rioja | 0 | 1.8 | ||||||||||
| Ciudad Real | 0.3 | 1.9 | ||||||||||
The incidence of skin cancer (cutaneous melanoma and NMSC) rose progressively between 1978 and 2007, with a greater rise of melanoma in females and a greater rise of NMSC in males. It should be noted, however, that a decline in world melanoma incidence was observed in the last period (2004-2007). The number of new cases of lip cancer also declined over the 30 years analyzed.
Nonmelanoma Skin CancerBCC has a higher incidence than SCC, with a ratio of approximately 4:1 cases based on standardized rates.27 The lifetime risk of BCC is estimated at 28% to 33%, compared with 7% to 11% for SCC.28
Compared with other populations around the world, people of European origin living in Zimbabwe had the highest rates of NMSC. The highest number of new cases was registered for the period 1988 to 1992 and corresponded to 535.4 cases per 100000 population for males and 343 for females. The corresponding rates for the African population in Zimbabwe during the same period were 80 times lower (3.8 in males and 4.1 in females), providing further support for the association between low Fitzpatrick skin type and an increased risk of NMSC.
Goiânia, Brazil had the highest incidence rates for NMSC in both sexes during the last 3 periods of the study (1993-2007), with rates increasing from one period to the next. Goiânia is located in midwestern Brazil and has very high UV index readings in autumn and winter and extremely high readings in spring and summer. The area has a tropical climate, and people dress accordingly in light clothing that leaves a considerable proportion of their body exposed to direct sunlight and a high risk of sunburn.29 These factors, combined with unhealthy sun habits and a general lack of awareness about the risks of sun exposure, result in inadequate sun protection, possibly explaining the rising incidence of NMSC in Brazil.30
Although NMSC cases are on the rise, reporting is still incomplete in many cancer registries due to the difficulty of systematically compiling information on this form of skin cancer, which largely affects elderly patients, follows an indolent course, and has few symptoms. Many patients therefore do not seek medical attention and may even be treated without a clinical or histologic diagnosis. In addition, because NMSC is easy to treat and has a favorable prognosis, many patients are seen outside hospital settings, which are less accessible to cancer registries. Finally, it is difficult to estimate the incidence of NMSC from death records due to the low mortality of this form of skin cancer.18,31–33 Because of this underregistration, thus, melanoma incidence rates may be higher in some countries than NMSC rates.
In Europe, following the recommendations of the IARC and the European Network of Cancer Registries, some registries only count the first NMSC a patient develops. In other words, just 1 case is reported per patient, even if the patient develops more than 1 primary NMSC (at the same time or later) or experiences recurrence.
It is therefore accepted that NMSC registration is incomplete and that this is an impediment to the establishment of accurate incidence rates.32,33
More and more cancer registries in Spain are collecting data on NMSC. Our analysis of individual registries in Spain showed that, with some exceptions observed for the last period (2003-2007), the number of new cases of NMSC is on the rise in both males and females.
Cutaneous MelanomaRates of melanoma around the world have increased faster than those of other cancers.14 The annual increase in new cases in the white population has been estimated at between 3% and 7%.4
Australia had the highest rates of melanoma for both males and females throughout the study period except for the last 5 years (2003-2007), when it was overtaken by the white population of Hawaii.
Australia has the highest reported incidence of melanoma in the world, with reports of between 30 and 60 new cases a year per 100000 inhabitants and even higher rates in Queensland.3,4,14
The main risk factor in Australia is high exposure to UV radiation from the sun. In 2010, 7220 cases of melanoma (4668 in men and 2552 in women), approximately 63% of all melanoma cases registered that year, were attributed to UV radiation.34
The decline in incidence noted for 2003 to 2007 suggests that the primary prevention campaigns implemented in this country over the previous 30 years, together with the banning of tanning booths, may have led more recent birth cohorts to modify their sun protection behaviors. Monitoring of incidence rates over the coming years will tell whether this trend is maintained or not.34
The fact that the white population of Hawaii had the highest melanoma rates in the last period of the study (2003-2007) is worthy of mention. Most white people in Hawaii are immigrants from the United States and many of them have constitutionally distinctive characteristics, such as Celtic or English ancestry, a fair complexion, freckles, susceptibility to sunburn, inability to tan, and a family history of skin cancer. These characteristics, together with residence in Hawaii for numerous years and long hours spent in the sun, have been directly linked to a high risk of cutaneous melanoma in men.35
In Europe, the highest melanoma rates registered for both sexes in the last study period, 2003 to 2007, were in Tyrol, Austria. Tyrol is an Alpine region whose inhabitants have fair skin (Fitzpatrick skin types i-iii) and live in extensive rural areas, with limited access to skin cancer screening programs. In addition, because of the high altitudes, they are exposed to relatively high doses of UV radiation.36,37
An increasing number of cancer registries in Spain are recording melanoma cases. Melanoma rates saw a progressive rise over the 30-year study period in all regions analyzed except Girona and Tarragona.
Although data are more complete for melanoma than for NMSC in cancer registries, melanoma incidence is still believed to be underregistered, partly because some countries do not have cancer registries and base their estimations on death records and information from neighboring countries.20
Lip CancerLip cancer, which is recorded as a separate entity in cancer registries, has a lower incidence than other forms of skin cancer. It is the most aggressive form of skin cancer and has a higher rate of metastasis than cancers in other areas of the face. This is particularly true of commissural lesions, which are associated with lymph node involvement in an estimated 20% of cases.38 The main risk factors for lip cancer are exposure to UV radiation and smoking. Incidence is higher in men, partly because women use lip protection more frequently. Women who apply lipstick once a day or less, for example, have been found to have twice the risk of lip cancer as those who apply it twice a day.9
On analyzing the different cancer registries in Spain, we observed a decrease in lip cancer in both sexes between 1978 and 2007. The decline was more evident in males, and there was a tendency towards stabilization in females in Asturias, the Basque Country, and Tarragona. The lower incidence rates might be due to a reduction in the number of smokers and workers chronically exposed to UV radiation.
One limitation of our study is that we were unable to analyze data for the period 2008 to 2012 as the IARC takes 5 years to publish the data collected. Our data are therefore not as up to date as they could be, but considering the rigorous epidemiological and scientific context, this shortcoming is totally justified. Another limitation is related to the fact that not all the populations analyzed have cancer registries. Nonetheless, the sample of registries used by the IARC adequately reflects the epidemiological situation of skin cancer. Finally, information on clinical and histological subtypes of melanoma would have enriched our analysis, but this was not available.
It is crucial to understand how skin cancer incidence rates vary over time and to ensure that the necessary data are registered with the same rigor as for other cancers, as this will help to guide clinical practice and inform primary and secondary prevention campaigns. Unlike previous studies published by our group,39 this study also contemplated variations in lip cancer incidence over time, providing thus a broader and more complete view of the situation of skin cancer within WHO registries.
Although skin cancer rates continue to grow worldwide, the latest data from Australia showing a slight decrease in melanoma incidence are encouraging and highlight the need to continue to actively implement prevention campaigns to increase awareness among the general population of the risk factors associated with skin cancer and bring about behavioral changes.
Ethical DisclosuresProtection of humans and animalsThe authors declare that no tests were carried out in humans or animals for the purpose of this study.
Confidentiality of dataThe authors declare that no private patient data appear in this article.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: García EM, Arias-Santiago S, Serrano-Ortega S, Buendía-Eisman A. Evolución de la incidencia del cáncer de piel y labio durante el periodo 1978-2007. Actas Dermosifiliogr. 2017;108:335–345.