Meta-analyses have found evidence of a relationship between psoriasis and metabolic syndrome, but Latin American populations have not been included.
MethodologyWe performed a systematic review and meta-analysis of observational studies including adults with psoriasis and metabolic syndrome indexed in Medline, Scopus, SciELO, Google Scholar, Science Direct, and LILACS between 1980 and 2016. We computed pooled odds ratios (OR) with a random effects model and analyzed subgroups according to patient variables used in the studies.
ResultsFive studies with a total of 241 patients with psoriasis were found; 46.5% of the patients also had metabolic syndrome (pooled OR, 2.63; 95% CI: 1.11-6.23; P=.03). In studies using the Adult Treatment Panel III (ATP-III) criteria for metabolic syndrome, the pooled OR was similar at 3.97 (95% CI: 1.27-21.42). Studies that included patients with chronic and severe disease detected higher risk for metabolic syndrome (pooled OR, 6.65; 95% CI: 3.32-13.31). Limitations are that few studies have been done in Latin America, heterogeneity was high, and inconsistency was found across studies.
ConclusionThe association between psoriasis and metabolic syndrome is high in Latin America. The association is stronger when psoriasis is chronic and severe and when the ATP-III criteria are used for diagnosis.
Diversos estudios de metaanálisis han demostrado la relación entre psoriasis y síndrome metabólico (SM), sin embargo, no incluyen población latina.
MetodologíaSe realizó una revisión sistemática y metaanálisis con trabajos observacionales incluidos en Medline, Scopus, SciELO, Google Scholar, Science Direct, LILACS, desde 1980 hasta 2016, sobre psoriasis y SM en adultos. Se halló el odds ratio combinado (ORC) con modelo de efectos aleatorios y se realizó un análisis por subgrupos según las variables de los estudios.
ResultadosSe incluyeron 5 trabajos, con 241 pacientes con psoriasis, y se encontró que el SM se presentó en el 46,5% del grupo de psoriasis, con un ORC de 2,63 (IC 95%: 1,11-6,23; p=0,03). Los estudios que utilizaron los criterios Adult Treatment Panel III (ATP-III) tuvieron una relación similar (ORC 3,97; IC 95%: 1,27-21,42), mientras que los que incluyeron pacientes crónicos y con enfermedad grave tuvieron un mayor riesgo de SM (ORC 6,65; IC 95%: 3,32-13,31). Entre las limitaciones del estudio tenemos la escasez de trabajos analíticos, la alta heterogeneidad, y la inconsistencia entre las publicaciones.
ConclusiónExiste una alta asociación entre psoriasis y SM en pacientes latinos, con mayor relación con cronicidad y gravedad de la enfermedad, y mejor desempeño de los criterios ATP-III para el diagnóstico de SM.
The chronic inflammatory skin disease psoriasis has a prevalence around 2% in Western countries1 and is recognized to be the most common immunologic disorder. The prevalence is 2.5% in Peru.2 Severity ranges from mild forms affecting small areas of the body to serious disease causing lesions extending over large areas and greatly affecting patients’ quality of life.3
Various cardiovascular risk factors have been associated with psoriasis in recent years, attributable to chronic systemic inflammation that leads to atherosclerosis and finally cardiovascular disease.4 Metabolic syndrome, which doubles the risk of coronary disease, offers a way to assess a patient for cardiovascular disease.5 This syndrome involves a constellation of conditions (obesity, dyslipidemia, diabetes mellitus, and hypertension) that have a greater effect on cardiovascular disease when they occur together than when they develop in isolation. About 25% of Latin Americans (17% in Peru) have metabolic syndrome 17%.6 Insulin resistance and abnormal functioning of adipose tissue are thought to be the underlying mechanism.7
Recent meta-analyses have found that patients with psoriasis are at risk for metabolic syndrome, with odds ratios (ORs) between 1.84 and 2.268) and that severe psoriasis puts patients at greater risk (OR, 1.98; 95%CI, 1.62–2.43) than mild forms of the disease (OR, 1.22; 95%CI, 1.11–1.35).9 The analyzed studies have not included Latin American populations, however.
The prevalence of metabolic syndrome in patients with psoriasis in Latin America ranges from 40% in Mexico10 to 70% in Honduras.11 In Peru the prevalence is 46%.12 Few original studies have addressed this question in this part of the world, however.
Consequently, although current recommendations suggest we screen for metabolic syndrome in patients with psoriasis, the strength of association between the 2 conditions has not been studied systematically in Latin America.
We undertook a meta-analysis with the main objective of assessing our current understanding the association between metabolic syndrome and psoriasis in Latin American patients.
MethodsWe followed the MOOSE reporting guidelines (for Meta-analysis of Observational Studies in Epidemiology)13 to design a systematic review of the literature. Included in the search were observational studies of various types: retrospective and prospective, cross-sectional, and case–control or cohort designs. We sought studies published between January 1980 and January 2016. Included studies had to assess the association between psoriasis and metabolic syndrome diagnosed by physical examination following predefined criteria or by a review of medical records. Studies also had to have 2 arms (psoriasis patients and controls) and report the incidence or prevalence of metabolic syndrome in each arm. Articles could be published in either English or Spanish.
Population characteristics were men or women aged 18 years or older who were native to any Latin American country and who had been diagnosed with psoriasis based on specific criteria. We excluded studies for which full datasets were unavailable even if we made the effort to contact authors.
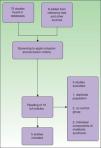
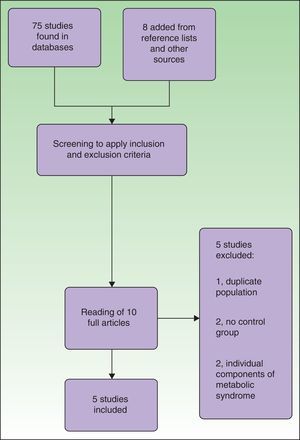
The literature search was carried out in MEDLINE, Scopus, SciELO, Google Scholar, Science Direct, LILACS and the virtual libraries of universities. The following search terms were used: ([psoriasis] or [vulgar psoriasis]) and ([metabolic syndrome] or [metabolic syndrome X]) and ([insulin resistance syndrome X] or [syndromeX, metabolic] or [metabolic X syndrome]). The search string was adapted to the syntax of each database. The initial search yielded 1493 titles. After eliminating studies not done in Latin American populations, we were left with 75 articles. Eight additional studies were identified by checking the references lists of the 75 indexed studies and from other sources of information. Two authors (M.R. and F.C.) screened the titles and abstracts of the 83 candidate studies to determine whether they seemed to meet the inclusion criteria. Ten were initially chosen but after careful review, 5 of them were excluded because 2 had no control group, 2 studied individual components of metabolic syndrome, and 1 used a population already included in another study. Thus, 5 articles met the inclusion criteria for systematic review and meta-analysis (Fig. 1). If the screening authors did not agree on whether to include or exclude an article, the third author (E.Q.) was consulted to resolve the disagreement.
The following data were gathered into a model spreadsheet in MS Excel for analysis: year of publication, country where the patients lived, design, number of participants in each group, mean age, prevalence of metabolic syndrome in each group, and adjustment for covariates. Authors M.R. and F.C. checked data twice for accuracy; discrepancies were resolved in consultation with E.Q.
Metabolic syndrome was defined in accordance with specific criteria. According to the Third Report of the National Cholesterol Education Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (the Adult Treatment Panel III [ATP-III]),14 metabolic syndrome is defined by the presence of 3 out of 5 of the following factors: 1) high blood pressure of at least 130/85mmHg (or on antihypertensive medication), 2) high fasting blood sugar level of 100mg/dL or higher (or on medication to lower sugar levels), 3) triglyceride level of 150mg/dL or higher (or on medication to lower the level), 4) a high-density lipoprotein level of 40mg/dL or less in men or 50mg/dL or less in women (or on therapy to raise that level), and 5) waist circumference of 102cm or more in men or 88cm or more in women.
Similar criteria were proposed by the International Diabetes Federation (IDF),15 but they named obesity as a necessary condition, defined by a body mass index greater than 30kg/m2 and specifying waist measurements for different ethnic groups. Although measurements for a Latin American population were not given, the IDF advised using those for a South Asian population (waist circumference of 90cm in men and 80cm in women.
The main outcome measure was the association between psoriasis and metabolic syndrome, reflected by an OR and 95% CI. We also calculated pooled ORs and 95% CIs by means of a random effects model. Significance was set at P<.05. Results for subgroups formed according to patient characteristics and criteria used to define metabolic syndrome were also analyzed. Heterogeneity between studies was measured with the I2 statistic. A value for I2 greater than 50% indicated heterogeneity.16 Descriptive statistics (percentages and means) were tabulated for the included studies; they were analyzed and figures created with the Stata13 program and Review Manager Version 5.1
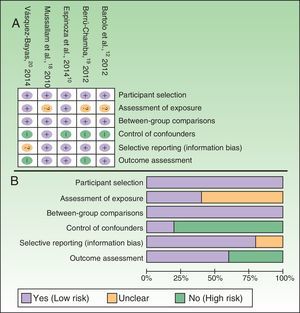
The quality of studies was evaluated with a modified version of the Cochrane risk of bias tool for observational studies. The tool lists 6 traits to score as 0 (absent) or 1 (present). A total score of 3 or less indicated low quality and a score of 4 or 5 indicated high quality, as described elsewhere.17 These scores were used in the subgroup analysis.
Publication bias was measured by constructing funnel plots. A symmetric, inverted funnel indicated a high probability that no publication bias was present.
Finally, sensitivity was assessed by removing a study to test its effect on the pooled OR. Similarity between pooled ORs with and without the article removed would indicate a high degree of certainty of results.
ResultsWe included 5 trials from 3 Latin American countries (Table 1). The trials analyzed 545 patients (241 with psoriasis and 304 controls). The meta-analyzed sample included 159 patients who had a recent diagnosis of psoriasis or who had not received systemic treatment (vs 218 controls); 82 patients with chronic psoriasis or who were on a systemic treatment were also included (vs 86 controls). All the trials were prospective case–control studies The ATP-III criteria for metabolic syndrome were applied in 2 of the studies,10,12 and the IDF diagnostic criteria were applied in the others18–20 (Table 1).
Characteristics of the Studies Included in the Meta-analysis and the Patients Enrolled.
| Study | Country | Period | Study Design | Patient Type (Severity, Treatment) | Metabolic Syndrome Diagnostic Criteria | Quality of Design | Patients Studied | Mean Age, y | Metabolic Syndrome | Pooled OR (95% CI)a | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Pso, n (%) | C, n (%) | Pso | C | n (%) | Pso, n (%) | C, n (%) | ||||||||
| Berrú-Chamba,19 2012 | Ecuador | July–Sept 2011 | C–C, prospective | PASI >0, all Pso patients | IDF | Low | 66 | 31 (47) | 35 (53) | NR | NR | 25 (38) | 18 (58) | 7 (20) | 5.5 (1.65–19.39) |
| Espinoza et al.,10 2014 | Mexico | Feb 2010–Feb 2011 | C–C, prospective | PASI >0, Pso de novo or no ST | ATP-III | High | 209 | 103 (49) | 106 (51) | 48 | 48 | 64 (31) | 43 (42) | 21 (20) | 2.9 (1.50–5.67) |
| Mussallam et al.,18 2010 | Peru | Jan–Dec 2009 | C–C, prospective | PASI >0, all Pso | IDF | High | 102 | 51 (50) | 51 (50) | NR | NR | 43 (42) | 33 (65) | 10 (20) | 7.5 (2.82–20.60) |
| Vásquez-Bayas,20 2014 | Ecuador | Oct–Dec 2014 | C–C, prospective | PASI >0, Pso de novo or no ST | IDF | Low | 96 | 32 (33) | 64 (67) | 45.3 | 45.8 | 30 (31) | 7 (22) | 23 (36) | 0.49 (0.15–1.43) |
| Bartolo et al.,12 2012 | Peru | Feb 2010–Jan 2011 | C–C, prospective | PASI >0, Pso de novo or no ST | ATP-III | High | 72 | 24 (33) | 48 (67) | NR | NR | 25 (35) | 11 (46) | 14 (29) | 2.05 (0.65–6.36) |
Abbreviations: ATP-III, Third Report of the National Cholesterol Education Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (the Adult Treatment Panel III)22; C, controls; C–C, case–control; Dec, December; Feb, February; IDF, International Diabetes Federation; Jan, January; NR, not reported; Oct, October; OR, odds ratio; PASI, Psoriasis Area and Severity Index; Pso, psoriasis; Sept, September; ST, systemic treatment.
Only pooled ORs are listed in the column because only 1 study (Mussallam et al.18) calculated an OR (7.5; 95%CI, 3.1–18.5) adjusted for obesity, hypertension, diabetes mellitus, and dyslipidemia.
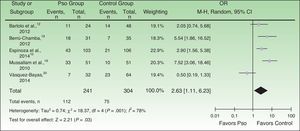
Metabolic syndrome was diagnosed in 46.5% in the psoriasis arm and in 24.7% of the controls (pooled OR, 2.63; 95%CI, 1.11–6.23; P=.03; I2=78%) (Fig. 2).
Forest plot of the prevalence of metabolic syndrome in psoriasis patients in the meta-analyzed observational studies. A Mantel-Haenszel (M-H) fixed-effect method was used to calculate the ORs and 95%CIs. Pso refers to psoriasis at any level of severity or point in diagnosis; OR, odds ratio.
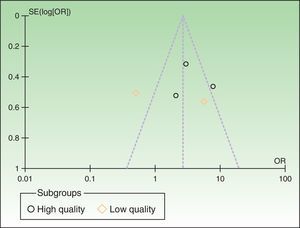
The funnel plots revealed asymmetry along the mean, suggesting high likelihood of publication bias (Fig. 3).
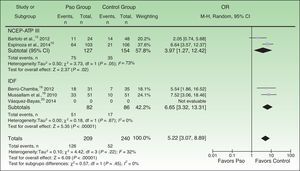
The subgroup analysis showed that the association between metabolic syndrome and psoriasis was stronger in the studies that used the ATP-III criteria (pooled OR, 3.97; 95%CI, 1.27–12.42) than in those that used the IDF criteria (pooled OR, 2.75; 95%CI, 0.50–15.27). Likewise, studies that included patients recently diagnosed with psoriasis, or not yet prescribed systemic treatment (psoriasis de novo subgroup), detected a significantly lower risk of metabolic syndrome (pooled OR, 1.50; 95%CI, 0.52–4.30) than those with only patients with chronic disease or treated with systemic drugs (psoriasis-in-treatment subgroup) (pooled OR, 6.65; 95%CI: 3.32–13.31) (Fig. 4).
Forest plots of the prevalence of metabolic syndrome in subgroups of patients with psoriasis formed according to patient characteristics. A Mantel-Haenszel (M-H) fixed-effect method was used to calculate the ORs and 95%CIs. Pso refers to psoriasis at any level of severity or point in diagnosis; OR, odds ratio.
Finally, analysis by quality of research design showed that risk of metabolic syndrome in psoriasis patients was higher in high-quality studies (pooled OR, 3.48; 95%CI, 2.22–5.45) than in low-quality studies (pooled OR, 1.44; 95%CI, 0.75–2.77). Fig. 5 shows the results of an analysis of risk of bias in these studies.
Analysis of sensitivity (Table 2) showed that the results for effect direction and size were not significantly affected by removing any study's data from the meta-analysis, suggesting the overall findings are robust.
Analysis of Sensitivity of the Meta-analysis to Study Removals.
| Study Removed | Pooled OR (95%CI) | I2, % | P |
|---|---|---|---|
| No removal (all studies) | 2.63 (1.11–6.23) | 78 | .030 |
| Mussallam et al.,18 2010 | 2.02 (0.81–5.06) | 76 | .130 |
| Vásquez-Bayas,20 2014 | 3.84 (2.20–6.69) | 37 | .000 |
| Berrú-Chamba,19 2012 | 2.22 (0.81–6.10) | 82 | .120 |
| Espinoza et al., 201410 | 2.56 (0.75–8.73) | 84 | .130 |
| Bartolo et al.,12 2012 | 2.79 (0.95–8.17) | 83 | .060 |
This meta-analysis is the first to include data for 241 Latin American patients with psoriasis, who were compared to 304 control patients to explore the relationship between metabolic syndrome and psoriasis in this region. We found that patients with psoriasis have a 3-fold higher risk for metabolic syndrome, which was present in 47% of these patients versus 25% of the controls (Fig. 2). This level of risk is greater than that reported by Armstrong et al.,9 who found a pooled OR of 2.26 (95%CI, 1.70–3.01; I2=86%), or by Miller et al.,8 who calculated a pooled OR of 1.84 (95%CI, 1.2–2.8; I2>75%).
It is important to bear in mind that the rate of metabolic syndrome in Latin America is high (around 25%; range, 18.8% to 43.3%23) because of the rising prevalence of obesity and diabetes mellitus in a region whose nutritional situation is in transition.24 The control population in our analysis, which consisted mainly of patients hospitalized in large cities, had a metabolic syndrome prevalence of 24.7%. That figure is consistent with other Latin American statistics, supporting the validity of our data.
These results show that metabolic syndrome is even more common among Latin Americans with psoriasis than in European cohorts, where the pooled OR in a meta-analysis of 5 observational studies was 2.36 (95%CI, 1.53–3.67).9 Early screening for this syndrome is therefore key to preventing cardiovascular complications, particularly in Latin America.
The heterogeneity analysis showed a high degree of variability between studies (I2=78%) (Fig. 2). Differences in age ranges and gender distributions in the studies and the failure to calculate adjusted ORs controlling for those variables account for the variability. In addition, the studies enrolled patients with different levels of psoriasis severity and used different diagnostic criteria for metabolic syndrome. One study reported findings that countered the trend. The results of our meta-analysis, therefore, should be interpreted cautiously. In fact, however, previous meta-analyses on this topic have met with similar limitations.
Nonetheless, our sensitivity analysis supports the robustness of our results, given that none of the exclusions we explored changed the original findings significantly (Table 2). One study, however, found evidence that contradicted the general trend.20
Finally, we detected a high risk of publication bias, demonstrated by asymmetry in the funnel plots (Fig. 3).
Biological Basis for the Association Between Metabolic Syndrome and PsoriasisVarious biological mechanisms account for the association between metabolic syndrome and psoriasis.25 The activation of T cells in psoriasis leads to the release of proinflammatory cytokines such as tumor necrosis factor (TNF), interleukin 2, and interferon gamma.26 These in turn induce the release of more cytokines and the expression of adhesion molecules, nitric oxide synthase, interleukin 6, and angiotensin II.27,28 TNF also mediates insulin resistance by favoring the release of free fatty acids, inhibiting the tyrosine kinase activity of the insulin receptor, and also modulating adipogenesis by suppressing adiponectin secretion from adipocytes29 while increasing leptin secretion.30,31 In this process, insulin-like growth factor II levels increase, promoting atherosclerosis, epidermal proliferation, hyperlipidemia, and diabetes mellitus.32 In addition, TNF leads to endothelial damage by promoting increased expression of adhesion molecules and atheromatous plaque formation. Plasminogen activator inhibitor 1 levels also rise, favoring thrombosis.33 Adipose tissue, a key participant in obesity disorders and metabolic syndrome, liberates multiple proinflammatory cytokines24 that are responsible for insulin resistance and endothelial dysfunction, similar to the process in psoriasis. Thus, the components of chronic inflammation, angiogenesis, and epidermal proliferation associated with psoriasis favor diabetes mellitus, thrombosis, and atherosclerosis alike.34 The proinflammatory state that develops in obesity, diabetes mellitus, and atherosclerosis increases susceptibility to psoriasis or exacerbates its severity.35,36 In addition, the elevated plasma renin and angiotensin converting enzyme activity37 of patients with psoriasis is implicated in the pathogenesis of hypertension in metabolic syndrome along with the presence of angiotensinogen, resistin, and leptin secretion by adipose tissue.38
Part of the hereditary aspect of the association between metabolic syndrome and psoriasis would derive from the fact that both psoriasis and diabetes mellitus share the cyclin-dependent kinase 5 regulatory subunit-associated protein 1-like gene locus.39 Psoriasis susceptibility genes (PSORS 2, PSORS 3, and PSORS 4) are also associated with greater susceptibility to metabolic syndrome, diabetes mellitus, dyslipidemia, and cardiovascular disease.40
Analysis According to Diagnostic Criteria for Metabolic SyndromeWe found that the studies that used the ATP-III criteria reported stronger associations between metabolic syndrome and psoriasis than studies using the IDF criteria. However, the study by Vásquez-Bayas 201420 clearly had a strong influence on those results, as their data went counter to the general trend and could potentially confound interpretation. After removing that study from the meta-analysis, the pooled OR for the other studies that used the IDF diagnostic criteria was much higher (6.65; 95%CI, 3.32–13.31) than the studies using the ATP-III criteria (pooled OR, 3.97; 95%CI, 1.27–21.42). Both ORs were statistically significant (Fig. 6).
Forest plot of the prevalences of metabolic syndrome in subgroups based on the diagnostic criteria (ATP-III or IDF) applied. The 2014 study by Vásquez-Bayas20 was excluded from this analysis because its results ran counter to the trend. Pso refers to psoriasis at any level of severity or point in diagnosis; OR, odds ratio; NCEP-ATP III, Third Report of the National Cholesterol Education Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (the Adult Treatment Panel III)22; IDF, International Diabetes Federation.
For a diagnosis of metabolic syndrome, the IDF criteria require the presence of obesity (defined by a waist circumference of at least 90cm in men and 80cm in women). The obesity criterion is optional in the ATP-III criteria, on the other hand, and is defined by a waist circumference of at least 102cm in men and 88cm in women.
Thus, the IDF criteria21 stipulate lower obesity thresholds than the ATP-III criteria and, although obesity is a required factor, the IDF criteria can be expected to classify more patients as having metabolic syndrome.41 We therefore suggest that future studies on the association between metabolic syndrome and psoriasis should apply the ATP-III criteria, given consensus among some authors that these offer the most useful tool for assessing vascular damage and cardiovascular events in nondiabetic persons.42 Moreover, they define specific criteria for Latin American populations.
Psoriasis Severity and Metabolic SyndromePatients with psoriasis who were not on systemic treatments or recently diagnosed were at lower risk for metabolic syndrome than patients with chronic psoriasis who were on systemic drugs (Fig. 6). It is therefore reasonable to conclude that studies in chronic patients with severe disease found they were at greater risk for metabolic syndrome compared to recently diagnosed patients whose disease was mild.
Several studies have found that psoriasis severity bears a directly proportional association with metabolic syndrome.43–45 Armstrong et al.9 found a dose–response relationship in a meta-analysis that calculated pooled ORs of 1.22, 1.56, and 1.98 for mild, moderate, and severe psoriasis, respectively.
The basis for this observation can be found in a recent report that patients with severe psoriasis have higher expression of inflammatory markers such as p-selectin and platelet-derived microparticles.46 Microparticles have been said to contribute to accelerated atherogenesis in psoriasis.47 Cell membrane vesicles contain nucleic acids and inflammatory mediators such as interleukin 1, CD40, and intercellular adhesion molecule 1 that are released after cell activation or apoptosis and contribute to vascular inflammation, thrombosis, and angiogenesis.48
LimitationsThe limitations of this meta-analysis derive from the scarcity of published studies and the inconsistency of design and reporting in those that are available. A large proportion of publications on the association between metabolic syndrome and psoriasis in Latin America are descriptive, making meta-analysis of data impossible. This problem of few qualitative studies reporting only descriptive statistics has been encountered in previous meta-analyses. We were able to include 5 studies from 3 Latin American countries. Although they are not representative of the entire geographic area of interest, the sample does allow us to extrapolate to obtain important information that warns of high rates of metabolic syndrome in our psoriasis patients.
The studies we included also differed in the diagnostic and inclusion criteria they applied, and they generally failed to report ORs adjusted for demographic variables such as age and sex. It is necessary to improve future studies by unifying design and analysis.
ConclusionsThis systematic review and meta-analysis shows that risk for metabolic syndrome is nearly 3-fold higher in Latin American patients with psoriasis than in the general population. Furthermore, the risk is higher for patients with severe or chronic psoriatic disease. Finally, we advise using the ATP-III criteria in future studies on this subject.
Clinical ApplicationOur findings stress the importance of screening psoriasis patients for metabolic syndrome and forming multidisciplinary treatment teams, particularly in Latin America. Patients should be enrolled in programs to detect cardiovascular risk factors; control weight, diet, and lifestyle; and treat obesity, diabetes mellitus, hypertension and dyslipidemia early. Clear clinical improvement of psoriasis after weight reduction through dieting and other means and specific adjuvant treatments has been demonstrated.49 Patients should be encouraged to adopt a healthy diet and engage in regular physical exercise.
Implications for ResearchNew studies are still needed to help us understand the strength of the association between psoriasis and metabolic syndrome in Latin America as well as to clarify the relation to cardiovascular events. Future studies applying better prospective designs and unified criteria are therefore planned. Furthermore, we propose wider use of the ATP-III diagnostic criteria for metabolic syndrome in the interest of greater consistency of results and because those criteria, which are specifically applicable to Latin American ethnic groups, are better able to predict cardiovascular risk.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Data confidentialityThe authors declare that no private patient data are disclosed in this article.
Right to privacy and informed consentThe authors declare that no private patient data are disclosed in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Rodríguez-Zúñiga MJM, Cortez-Franco F, Quijano-Gomero E. Relación entre psoriasis y síndrome metabólico en Latinoamérica. Revisión sistemática y metaanálisis. Actas Dermosifiliogr. 2017;108:326–334.