Capecitabine, a prodrug of 5-fluorouracil (5-FU), is a chemotherapeutic agent that is administered orally. It is absorbed in the intestine and subsequently undergoes a series of enzymatic transformations via the thymidine phosphorylase pathway to produce the biologically active metabolite 5-FU.1
Recent years have seen an increase in capecitabine use, mainly for the treatment of cancer of the gastrointestinal tract and breast, owing to its oral route of administration and its better toxicity profile compared with 5-FU, which is associated with a higher incidence of serious adverse effects. The most frequent cutaneous adverse effect of capecitabine is palmoplantar erythrodysesthesia.2 Cases of cutaneous lupus (mainly the subacute form) have also been associated with capecitabine treatment.
We present the case of a 62-year-old man who was referred from the oncology to the dermatology department for assessment of pruritic skin lesions on the face and chest that had appeared 2 months earlier. His personal history included high blood pressure, dyslipidemia, and hyperuricemia, for which he had been receiving the same treatment for several years. The patient also had esophageal squamous cell carcinoma with bone metastasis, for which he had started capecitabine treatment 7 months earlier.
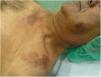
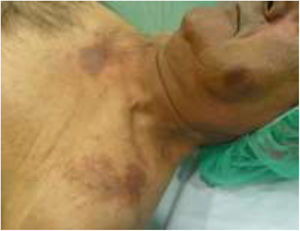
The physical examination revealed round erythematous plaques (approximately 3 cm) with focal desquamation located on the cheeks. Two larger erythematous–violaceous plaques were located on the upper chest (Fig. 1). No other lesions of interest were detected on the rest of the examined skin surface.
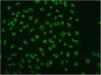
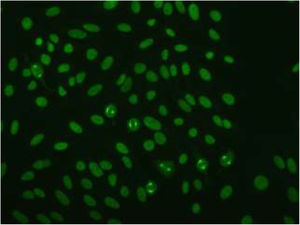
Based on the characteristics of the lesions, the following possible diagnoses were considered: cutaneous lupus, Sweet syndrome, fixed drug eruption, and cutaneous lymphoma. Blood tests and an immunological study revealed positive antinuclear antibodies (titer, 1:1280), with a NuMA-1-type fluorescence pattern (Fig. 2). The patient was negative for anti-DNA, anti-ENA (extractable nuclear antigen), and antihistone antibodies.
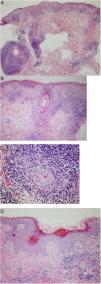
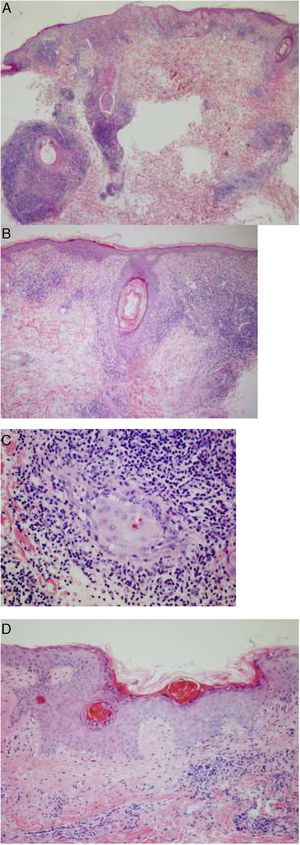
One of the lesions was biopsied for histology, which showed a dense inflammatory infiltrate in both the epidermis and the pilosebaceous follicles composed mainly of lymphocytes and histiocytes, with few plasma cells. Focal dyskeratotic keratinocytes were also identified. Areas with epidermal atrophy, parakeratosis, and thickening of the basement membrane were also evident, alternating with other areas with better preserved architecture. Moderate mucin deposits were observed in the reticular dermis (Fig. 3).
Histopathology of one of the lesions. A and B, Dense inflammatory infiltrate in the epidermis and pilosebaceous follicles. C, Infiltrate composed mainly of lymphocytes and histiocytes, with few plasma cells. D, Areas of epidermal atrophy, parakeratosis, and thickening of the basement membrane, alternating with other areas in which the architecture is better preserved.
Based on these findings a diagnosis of capecitabine-induced discoid cutaneous lupus was established. Capecitabine treatment was discontinued and replaced with the combination of carboplatin and placitaxel, which resulted in gradual improvement of the lesions and resolution 3 months later, leaving residual hyperpigmentation.
Drug-induced lupus erythematosus consists of the appearance of cutaneous signs compatible with lupus (both systemic and limited cutaneous forms), coinciding with the introduction of a new drug. This condition can be triggered by many drugs. Latency can range from a few weeks in some cases of subacute cutaneous lupus erythematosus to more than 6 months in chronic cases. Clinical signs usually resolve after discontinuation of the causative drug. The etiology remains unknown, although it is thought to be influenced by both individual susceptibility and metabolism of the drug in question.3
Drug-induced chronic cutaneous lupus erythematosus is an uncommon disease. It is characterized by erythematous squamous lesions that are located predominantly in photo-exposed areas and tend to resolve leaving an atrophic scar. This condition has been associated with capecitabine, 5-FU, and anti–tumor necrosis factor agents (adalimumab, infliximab).3
The immunological profile usually reveals positivity for antinuclear antibody (ANA) only. In our case, immunohistochemistry revealed a characteristic ANA fluorescence pattern known as NuMA-1. This is a rare ANA subtype (<1%) that targets a mitotic spindle protein, and is mainly associated with autoimmune diseases (e.g. Sjögren syndrome, systemic lupus erythematosus) and, to a lesser extent, infections and neoplastic processes.4
At least 9 cases of cutaneous lupus erythematosus associated with capecitabine treatment have been described to date,5–13 only one of which corresponds to discoid lupus (Table 1).
Cutaneous Lupus Associated With Capecitabine Treatment: Cases Published to Date
| Authors | Histological Findings | Immunological Findings | Diagnosis | Latency Period | Lesion Progression After Discontinuing Treatment |
|---|---|---|---|---|---|
| Weger et al5 2007 | Epidermal atrophy, interface dermatitis with cytolytic changes in basal layer keratinocytes, and marked mucinosis | Anti-Ro+ | Subacute cutaneous lupus | 7 d | Resolution in 6 wk |
| Merlin et al6 2008 | Epidermal atrophy, liquefaction of the basal layer, perivascular and perifollicular lymphoid infiltrate | ANA+ | Discoid cutaneous lupus | 3 mo | Resolution in 4 wk |
| Anti-DNA− | |||||
| Anti-ENA− | |||||
| Anti-Ro− | |||||
| Anti-La− | |||||
| Fernandes et al.7 2009 | Interface reaction, vacuolar degeneration of the basement membrane, dyskeratosis, perivascular lymphocytic infiltrate, and interstitial mucin deposition | ANA+ | Subacute cutaneous lupus | 2 wk | Resolution in 4–6 wk |
| Anti-histones + | |||||
| Anti-Ro+ | |||||
| Floristan et al8 2009 | Interface dermatitis with vacuolar degeneration of the basal keratinocyte layer | ANA+ | Subacute cutaneous lupus | 3 wk | Resolution in 2 wk |
| Anti-Ro+ | |||||
| Anti-histones− | |||||
| Kindem et al9 2013 | Epidermal atrophy with necrotic keratinocytes, vacuolar degeneration, perifollicular and perivascular lymphohistiocytic infiltrate in the dermis, and marked mucinosis | ANA+ | Subacute cutaneous lupus | 10 d | Resolution in 3 wk |
| Anti-Ro+ | |||||
| Anti-La− | |||||
| Anti-DNA− | |||||
| Anti-histones − | |||||
| Ko et al10 2013 | Interface dermatitis with basal hydropic changes, perivascular lymphocytic infiltrate, and interstitial mucin deposits | ANA+ | Subacute cutaneous lupus | 1 mo | Resolution |
| Anti-Ro+ | |||||
| Anti-La+ | |||||
| Fongue et al.11 2014 | Epidermal atrophy with focal necrosis of keratinocytes, thinning of the basement membrane, perivascular lymphocytic infiltrate, and mucin deposits in the reticular dermis | ANA+ | Subacute cutaneous lupus | 4 mo | Resolution in 3 wk |
| Anti-Ro+ | |||||
| Li et al12 2014 | Basal layer hydropic degeneration, perivascular lymphohistiocytic infiltrate, and abundant dermal mucin deposits | ANA+ | Subacute cutaneous lupus | 2 wk | Resolution in 12 wk |
| Anti-Ro+ | |||||
| Anti-La+ | |||||
| Kim et al13 2016 | Vacuolar degeneration of the basal epidermal layer, periappendicular lymphocytic infiltrate, and mucin deposition in the dermis | ANA+ | Subacute cutaneous lupus | 6 wk | Resolution |
| Anti-Ro+ | |||||
| Present case, 2018 | Epidermal and perianexial lymphohistiocytic infiltrate, dyskeratotic keratinocytes, and mucin deposition in the reticular dermis | ANA+ | Discoid cutaneous lupus | 5 mo | Resolution in 12 wk |
| Anti-DNA− | |||||
| Anti-ENA− | |||||
| Anti-histone− |
Abbreviations: ANA, antinuclear antibody; ENA, extractable nuclear antigen.
Therefore, although rare, capecitabine can induce discoid cutaneous lupus and this diagnosis should be suspected in patients who are treated with this drug and present a compatible clinical picture and concordant immunology and histology findings.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Moreiras Arias N, Rosón López ME, Suárez Peñaranda JM, Vázquez Veiga HA. Lupus cutáneo discoide inducido por capecitabina. Actas Dermosifiliogr. 2021;112:859–862.