As new antiangiogenic therapies have been introduced and added to the therapeutic arsenal against various types of cancer, previously unknown adverse effects have been detected. These effects negatively impact patients’ quality of life and can even make it necessary to suspend treatment. Adverse skin reactions occur in 90% of patients treated with angiogenesis inhibitors. In some cases, a correlation has been observed between the severity of reactions and treatment efficacy and tumor response. It is therefore extremely important that dermatologists be able to recognize and manage these reactions. Moreover, in order to avoid the unjustified withdrawal of potentially life-extending treatments, dermatologists must be able to differentiate between non-life-threatening reactions and life-threatening reactions that necessitate the suspension of treatment. In this review article, we analyze the main cutaneous adverse effects of the most common antiangiogenic agents.
Con el empleo de las nuevas terapias antiangiogénicas y su incorporación en el arsenal terapéutico de los distintos tipos de cáncer, se han detectado nuevos efectos adversos, no conocidos previamente, que afectan a la calidad de vida de los pacientes e incluso pueden obligar a suspender el tratamiento. Los efectos secundarios cutáneos ocurren en un 90% de los pacientes tratados con fármacos inhibidores de angiogénesis. En algunas ocasiones la severidad de las reacciones cutáneas se ha correlacionado con la eficacia y respuesta tumoral al tratamiento, por lo que resulta extremadamente importante para el dermatólogo ser capaz de reconocer y manejar este tipo de reacciones, diferenciar aquellas que pueden ser peligrosas para la vida del paciente y que obliguen a interrumpir el tratamiento de las que no lo son, evitando de esta forma la supresión no justificada de estas terapias que potencialmente pueden prolongar la vida del paciente. En esta revisión se analizan los efectos adversos cutáneos más importantes de los principales fármacos antiangiogénicos.
Several drugs that inhibit angiogenesis have produced encouraging results in recent years in the treatment of certain types of tumor. These drugs have opened the door to an important new approach to cancer management that will no doubt be a subject of further research in the future. However, skin toxicity affects around 90% of patients treated.1 Over 100 years ago it was observed that the growth of malignant tumors was associated with a proliferation of blood vessels.2 The hypothesis that tumor cells could produce proangiogenic substances was first proposed in 1968,3,4 and in 1971 Folkman5 published a hypothesis on the therapeutic potential of the inhibition of angiogenesis in cancer, marking the beginning of a new era in cancer research. The promising idea that the growth of malignant tumors and of their metastases could be inhibited by blocking the proliferation of blood vessels led to a vigorous search for pro- and antiangiogenic factors in the years following Folkman's publication. The result of those years of intense research is a number of molecules that inhibit angiogenesis, including sorafenib, sunitinib, and bevacizumab, which have been approved by the FDA for the treatment of various types of cancer, and many more molecules whose efficacy is currently being evaluated in clinical trials in different phases.
Sorafenib is a multikinase inhibitor approved by the FDA for the treatment of advanced renal cell carcinoma and hepatocellular carcinoma. It is administered by mouth and has a half-life of 25 to 48hours.6 Sorafenib inhibits numerous tyrosine kinases, including the family of vascular endothelial growth factor receptors (VEGFR-2 and VEGFR-3), platelet derived growth factor receptor (PDGFR), stem cell growth factor receptor (c-KIT), Fms-like tyrosine-kinase 3 (FLT3), and glial cell-line derived neurotrophic factor receptor encoded by the RET protooncogene, and also inhibits RAF kinases (RAF-1 and B-RAF).1
Sunitinib, likewise, is a multikinase inhibitor administered by mouth, with a half-life of 40 to 60hours. It also inhibits certain tyrosine kinases, such as VEGFR-1, -2, and -3, PDGFRα and PDGFRβ, colony stimulating factor 1 receptor (CSF1R), c-KIT, glial cell-line derived neurotrophic factor receptor (RET), and FLT3. Sunitinib has been approved by the FDA for the treatment of advanced refractory or metastatic gastrointestinal stromal tumors (GIST), cases of GIST with intolerance or resistance to treatment with imatinib mesylate, advanced or metastatic renal cell carcinoma, and unresectable or metastatic pancreatic neuroendocrine tumors.6 It has also been shown to be successful in the treatment of colon and breast carcinomas,7 and there have been reports of cases in which metastatic cutaneous adnexal tumors have stablized.8
Bevacizumab is a recombinant humanized anti-VEGF monoclonal antibody (93% human, 7% murine), available since 2005 for the treatment of colorectal and breast carcinoma, nonsmall cell lung cancer, renal cell carcinoma, ovarian and fallopian tube cancer, and primary peritoneal cancer, in combination with other anticancer agents.9 It is administered by intravenous infusion and exerts its antiangiogenic effect by binding to all the isoforms of VEGF, causing a fall in the number of endothelial cells and in the number of microcapillaries in the tumor tissue, in addition to reducing in vascular permeability.10
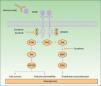
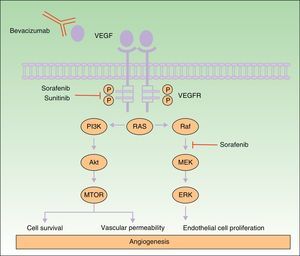
Many of the side effects of the antiangiogenic drugs are similar to those caused by traditional chemotherapeutic agents, including skin rash, mucositis, alopecia, xerosis, and pruritus.11 In this review we will focus on the adverse skin reactions most specific to the antiangiogenic drugs (Table 1). The different mechanisms of action of these 3 drugs are shown diagrammatically in Figure 1.
Summary of the Most Common Cutaneous Side Effects of the Angiogenesis Inhibitors.
| Drug | Side Effect | Frequency According to Different Series |
| Sorafenib | Hand-foot skin reaction | 21%-93% |
| Subungual splinter hemorrhages | 70% | |
| Erythematous rash on the face and scalp | 63% | |
| Scalp dysesthesia | 49% | |
| Alopecia | 4%-57% | |
| Skin rash | 15%-80% | |
| Pruritus | 4%-32% | |
| Xerosis | 6%-27% | |
| Spiny follicular hyperkeratosis | 21% | |
| Skin neoplasms and precancerous lesions | More than 50 cases | |
| Areolar hyperkeratosis or pain | 7 cases | |
| Eruptive nevi | 5 cases | |
| Psoriasiform rash | 3 cases | |
| Eruptive facial cysts | 2 cases | |
| Sunitinib | Facial edema | 24%-50% |
| Stomatitis | 36% | |
| Hand-foot skin reaction | 19%-36% | |
| Yellowish skin discoloration | 17%-30% | |
| Subungual splinter hemorrhages | 10%-25% | |
| Xerosis | 21% | |
| Skin rash | 13%-24% | |
| Change in hair color | 10%-13% | |
| Alopecia | 6%-12% | |
| Changes in the genital region | 12.5% | |
| Pyoderma gangrenosum | 5 cases | |
| Bevacizumab | Skin rash | 19%-46% |
| Delayed wound healing | 13% | |
| Ulceration of corticosteroid-induced striae | 4 cases | |
| Erythematous papular facial rash after intravitreous injection | 2 cases |
Mechanism of action of bevacizumab, sorafenib, and sunitinib. Diagram of the signaling pathways implicated in the process of angiogenesis and the sites of action of sorafenib, sunitinib, and bevacizumab. Activation of the vascular endothelial growth factor receptors affects angiogenesis by inducing cell proliferation, cell survival, and vascular permeability. Abbreviations: VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor.
Approximately 90% of patients treated with sorafenib will present cutaneous side effects.12 To date, there have been 5 main series evaluating cutaneous side effects in patients treated with sorafenib (Table 2).
Largest Series Evaluating Cutaneous Side Effects in Patients Treated With Sorafenib.
| Author and Reference | Year | Description |
| Autier et al.13 | 2008 | Prospective, double-blind study with 85 patients (43 in the sorafenib arm, 42 controls receiving placebo) |
| Lee et al.7 | 2009 | Sample of 109 patients treated with sorafenib |
| Robert et al.14 | 2009 | Pool of studies including clinical trials (4 phase 1, 1 phase 2, and 1 phase 3) |
| Kong et al.15 | 2009 | 65 patients: 24 in a prospective cohort and 41 in a consultation cohort |
| Zhang et al.12 | 2011 | Meta-analysis (6011 patients, systematic review of studies in Medline and Embase) |
Hand-foot skin reaction is one of the most common cutaneous side effects of sorafenib treatment and is detected in 34% to 78% of patients treated with this drug, depending on the series.12 The term hand-foot skin reaction (HFSR) is used to differentiate this type of lesion from classic hand-foot syndrome (HFS) and palmar-plantar erythrodysesthesia associated with traditional chemotherapy agents. Furthermore, this reaction is the skin toxicity most commonly associated with the discontinuation of sorafenib.13
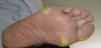
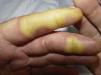
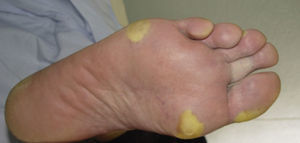
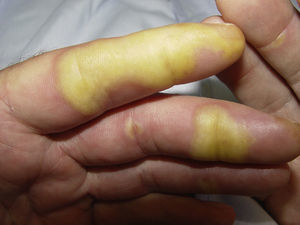
Clinically, HFSR develops within the first 2 to 6 weeks of treatment as hyperkeratotic lesions, with the formation of calluses and, in some cases, superficial blisters on an erythematous base, mainly affecting the palms and soles bilaterally, though calluses can also appear on the flexor surfaces of the fingers. The lesions usually have a characteristic peripheral erythematous halo (Figs. 2 and 3).14 This clinical presentation contrasts with the acute appearance of erythema, edema, paresthesia, pain, and subsequent diffuse peeling typical of classic HFS. Other symptoms associated with HFSR include paresthesia, a burning sensation, pain in the palms and soles, and reduced tolerance to contact with hot objects. These subjective changes usually precede appearance of the skin lesions and do not occur without the subsequent appearance of lesions. Symptoms are dose related and are noted in areas of skin subject to pressure or friction.16 The associated pain can have a major impact on quality of life and can interfere considerably with the patient's daily activities.17 A severe episode can require dose reduction, the temporary interruption of treatment, or even permanent withdrawal of the drug.
Hand-foot skin reaction. Hyperkeratotic lesions on the flexor surfaces of the fingers, with the formation of calluses and, in some cases, of superficial blisters on an erythematous base. The characteristic peripheral erythematous halo can be seen.
Figure courtesy of Dr Tuneu and Dr López Pestaña of Hospital Donostia, San Sebastian, Spain.
Histology reveals parakeratosis, dyskeratosis, and an intense, predominantly lymphocytic superficial perivascular inflammatory infiltrate. The most relevant feature is keratinocyte damage, with the presence of eosinophilic intracytoplasmic bodies, vacuolar keratinocyte degeneration, dyskeratotic keratinocytes, and confluent keratinocyte necrosis that leads to intraepidermal cleavage with the formation of intraepidermal blisters.18 There may be associated nonleukocytoclastic vasculitis.13 Immunohistochemistry has shown a loss of cytokeratin 10 and increased expression of cytokeratin 14, which would suggest an effect of the drug on keratinocyte differentiation.14
Jain et al.19 hypothesized that the clinical differences between HFS and HFSR could be explained by differences in the etiological and pathogenic mechanisms underlying the 2 conditions. One of the proposed mechanisms for the onset of hand-foot syndrome induced by traditional chemotherapy drugs is excretion of the chemotherapy agents via the sweat glands.11 This mechanism could not however be demonstrated for hand-foot skin reaction associated with multikinase inhibitors, as significant levels of sorafenib were not detected in the sweat of these patients.19 In this case, the authors proposed leakage of the drug through capillaries damaged by subclinical trauma as the pathogenic mechanism. The subsequent inhibition of VEGFR and PDGFR affects capillary repair and regeneration, interfering with tissue regeneration in these areas. This hypothesis is supported by the appearance of lesions of HFSR specifically in areas chronically exposed to friction and with preexisting hyperkeratosis.20 Recently, the existence of a genetic predisposition to develop sorafenib-induced HFSR has been described in patients with hepatocellular carcinoma.21 Dranitsaris et al.22 developed and validated a predictive model for the onset of HFSR in patients receiving treatment with sorafenib.23,24
The management of HFSR is summarized in Table 3.
Management of Sorafenib-Induced Hand-Foot Skin Reaction23,24
| Grade | Description | Recommendations | Dose Changes |
| Prophylaxis | Not applicable | Whole-body examinationElimination of callusesUse cotton socks and glovesAvoid hot water and tight footwear | Not applicable |
| 1 | Minimal skin changes or painless dermatitis | Avoid hot waterEmollient creamsThick cotton gloves and socksCream containing urea, 20%-40% | No. Maintenance of the same dose is recommended |
| 2 | Skin changes (peeling, blisters, hemorrhage, edema, hyperkeratosis) with a variable degree of pain.Interference with daily activities | As for grade 1Clobetasol cream, 0.05%Lidocaine, 2%Codeine, pregabalin for pain | YesReduce dose by 50% for 1-4 wkReintroduction of the full dose |
| 3 | Severe skin changes (peeling, blisters, hemorrhage, edema, hyperkeratosis) with painInterference with daily activities, including self-care | As for grades 1 and 2 | YesInterrupt treatment for 1 wk to reduce severity to grade 0-1Reintroduce at 50% of dose and then return to starting dose if no recurrence |
Source: National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03 (NCI-CTCAE v 4.03).24
In the prospective study by Autier et al.,13 an erythematous facial rash very similar to classic seborrheic dermatitis was observed in 63% of patients treated with sorafenib, compared with 2% of patients in the control group. It typically develops during the first 3 weeks after commencing treatment and resolves spontaneously in less than 2 months. In some cases, symptoms can be exacerbated by high temperatures. The pathogenic mechanism is unknown, but as no erythematous facial rash has been observed during treatment with sunitinib or imatinib, it is though that RAF inhibition rather than inhibition of VEGFR or PDGFR plays an important role.25 Histology reveals only a nonspecific lymphocytic infiltrate with no eosinophils or vasculitis. There is no correlation between the facial rash and the response to treatment. In the majority of cases, no treatment is required or only treatment similar to that for seborrheic dermatitis.13
Scalp DysesthesiaAs with the erythematous facial rash, scalp dysesthesia is a manifestation that usually develops between the first and third weeks of treatment. In the study by Autier et al.,13 scalp dysesthesia was observed in 49% of patients treated with sorafenib. In 30% of patients, scalp dysesthesia was the only skin manifestation. It tends to resolve spontaneously within days to weeks and does not usually require treatment.
Subungual Splinter HemorrhagesSubungual splinter hemorrhages are observed in around 70% of patients. Clinically, they present as painless black or red lines beneath the distal nail plate, most commonly affecting the fingernails, but also seen under the toenails. They usually appear within the first 2 months of treatment and resolve spontaneously with growth of the nail.13 In the past, these lesions have typically been associated with endocarditis or circulating anticardiolipin antibodies, which give rise thromboembolic phenomena. Splinter hemorrhages can also appear in healthy individuals after microtrauma, in which case they are usually limited to a single nail.14 When they develop in patients on treatment with sorafenib, it has been postulated that they occur as a result of a failure of regeneration of the capillaries due to VEGFR inhibition in areas subject to microtrauma.25
AlopeciaThe frequency of alopecia in patients treated with sorafenib varies according to the series, from 21% reported by Kong et al.,15 27% by Robert et al.,14 and 26% by Lee et al.,7 to 44% reported by Autier et al.13 In the meta-analysis by Zhang et al.,12 which included 18 clinical trials with 4444 patients treated with sorafenib, the overall incidence of alopecia was 25.5%. Sorafenib-related alopecia has sometimes been associated with slow beard growth14 and usually develops between 3 and 15 weeks after starting treatment. In the series by Autier et al.,13 19% of patients presented loss of body hair, not always associated with scalp alopecia. No specific treatment is available, but it is a transient condition and patients recover hair density after completing treatment, or sometimes earlier. Occasionally, when hair growth recovers, the hair can be curly and brittle.14
Skin Neoplasms and Precancerous LesionsSorafenib has been associated with the appearance of actinic keratosis, focal squamous atypia, keratoacanthoma, keratoacanthoma-like squamous cell carcinoma (SCC), and invasive SCC.26,27 These lesions are more common in men and are multiple in the majority of cases. They tend to arise in patients with no previous history of skin cancer, premalignant skin lesions, significantly photoaged skin, or of exposure to radiation in the affected area. These types of lesion have been found to appear between 2 weeks and 3 years after starting treatment. They typically occur in sun-exposed areas, although lesions in nonexposed areas have also been reported.28 Recently, Arnault et al.29 demonstrated that sorafenib produces paradoxical activation of the MAP kinase pathway, depending of the degree of activation, the duration of treatment, and the drug dose; this pathway induces cell proliferation, giving rise to the appearance of lesions that may be benign (cystic follicular lesions), borderline (keratoacanthomas), or malignant (SCC). Thus, although sorafenib is able to halt cell proliferation in solid tumors, it paradoxically stimulates the formation of skin tumors. It is thought that inhibition of the RAF kinase signaling pathway may have an additional effect on keratinocyte differentiation and proliferation.30 It is interesting that other multikinase inhibitors that share the same mechanism of action have not been associated with any increase in the risk of malignant and premalignant skin lesions. This difference probably arises because sorafenib is the only member of this group of drugs that inhibits RAF serine/threonine kinase. In favor of this hypothesis, it has been observed that suramine (a drug used to treat trypanosome infection and also, recently, to treat solid tumors), which has the same mechanism of action as sorafenib, inhibiting RAF kinase, also induces keratoacanthomas and SCC.31 In addition, vemurafenib, a selective RAF inhibitor employed in patients with metastatic melanoma carrying the BRAF V600E mutation, has also been found to increase the incidence of SCC and keratoacanthoma .29,32 It has been shown that cells of SCCs from patients treated with RAF inhibitors presented a different profile of mutations from typical SCCs. In the first group, mutations have been detected in MYC, FGFR3, and VHL for the first time, whereas mutations described previously in SCCs include TP53, CDKN2A, HRAS, KRAS, and PIK3CA.33 These findings may at least in part explain the rather atypical epidemiology and presentation of these premalignant and malignant lesions (such as the appearance of lesions in unexposed areas, rapid growth, multiple lesions, patients with no past history of skin cancer, etc.) in patients treated with sorafenib. Attention must be drawn to the differences between the characteristics of skin tumors induced by sorafenib and vemurafenib. The latest studies suggest that paradoxical activation of the MAPK pathway is because RAF blockade in cells carrying wild-type BRAF increases signaling via CRAF and ERK, and this would increase the MAPK pathway signaling, causing the appearance of SCCs. But this situation must also be linked to the presence of RAS mutations in keratinocytes—possibly produced by sun exposure or by viral infection—and the 2 factors together would form the mechanism of activation of MAPK signaling for tumors to develop.30,34 Treatment with vemurafenib is associated with a higher frequency of appearance of SCCs and keratoacanthomas (20%-30% of patients) than sorafenib (6%-7% of patients) and, in addition, there is a shorter latency period between the initiation of treatment and the appearance of the secondary skin tumors compared with sorafenib.29,34 This difference probably occurs because, although sorafenib has pan-RAF inhibitory properties, it has a much lower potency against the different RAF isoforms than selective inhibitors such as vemurafenib.29,33 SCCs induced by either drug are usually well-differentiated lesions with a good prognosis. No cases of metastasis or local recurrence have been reported after the surgical excision of SCCs in patients treated with these RAF inhibitors.30
In order to ensure an early diagnosis and adequate treatment of these lesions, whole-body examination of patients is recommended before starting treatment, with periodic revisions during treatment. Actinic keratosis must be treated early to avoid rapid progression to SCC. Sufficient scientific evidence exists to recommended photoprotection to patients who are on or are going to start treatment with sorafenib.29 Although the primary objective is to treat the initial tumor that threatens the life of the patient, it must not be forgotten that the risk of metastasis from an SCC, particularly in these patients who are in a state of immunosuppression, is relatively high. Furthermore, it has been reported that SCCs in these patients show a faster progression, and the early diagnosis and adequate treatment of these lesions is therefore extremely important. It has been demonstrated that patients who continue treatment develop more lesions, whereas 90% of those who discontinue the treatment do not develop further lesions or recurrence of previous lesions.11 When a patient on treatment with sorafenib is diagnosed with SCC, a short interruption of treatment or a dose reduction may be contemplated until the tumors can be excised; if another effective drug is available, a change should be considered. In those patients in whom sorafenib administration was achieving good results, treatment can be continued after all lesions have been treated, but special attention must be paid to examination of the whole skin and strict sunscreen protection must be employed.28
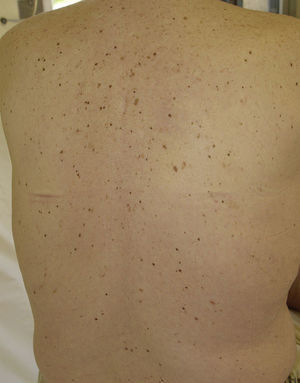
Eruptive Melanocytic LesionsThere have been reports of the eruptive appearance of melanocytic lesions in patients treated with sorafenib (Fig. 4). Kong et al.35 described 2 patients in whom multiple pigmented lesions appeared; the histological diagnosis was melanocytic nevi in 1 patient and lentigines in the other. In the first case, a number of melanocytic nevi appeared on the palms and soles after 2 months of treatment with sorafenib. In the second case, lentigines appeared on the neck, trunk, thighs, and palms and soles a month after commencing treatment. Bennani-Lahlou et al.36 described 5 cases of the appearance of hundreds of small (1 to 3mm) homogeneous dark-brown nevi after a mean of 9 months of treatment with sorafenib. The lesions arose on the trunk and upper limbs. This phenomenon has been attributed to the state of immunosuppression and inflammation. It has been reported in association with classical chemotherapy treatments and other situations of immunosuppression.35 The paradoxical appearance of atypical melanocytic lesions, and even melanomas, has also been reported in association with vemurafenib, a V600E BRAF inhibitor used to treat advanced or metastatic melanoma. It is thought that this phenomenon is due to paradoxical activation of the RAF-MEK-ERK pathway by CRAF.37,38
Spiny Follicular HyperkeratosisIn 2009, López et al.39 were the first to report the appearance of keratotic follicular papules on the face of a patient 10 days after starting treatment with Sorafenib; the lesions measured 1mm and were of normal skin color. Histology revealed marked follicular hyperplasia, particularly in the region of the follicular isthmus, with acanthosis, regenerative changes with numerous mitotic figures, occasional apoptotic cells, and a mild perifollicular lymphocytic infiltrate. The authors stated that all the lesions disappeared after the interruption of treatment with sorafenib. Franck et al.40 studied 43 patients on treatment with sorafenib. In 9 patients (21%) they found spiny skin lesions of 0.5mm to 1mm in diameter and 5mm in height, to which they gave the name spiny follicular hyperkeratosis. The lesions occurred on the face, scalp, and trunk, while the palms and soles were spared. The lesions appeared a mean of 82 days after starting treatment with sorafenib and resolved 98 days after the discontinuation of treatment. In 4 of the patients, sorafenib had to be withdrawn for other reasons and the rash disappear in 5 to 7 days. In 2 patients who recommenced treatment, the lesions reappeared. The most noticeable histologic finding was the presence of an orthoparakeratotic column that filled the infundibulum and protruded above the epidermal surface. Joncas et al.41 described the case of a patient with a similar rash of spiny hyperkeratotic follicular lesions on the scalp, trunk, and abdomen; the patient also presented alopecia of the scalp and eyebrows and a painful papulopustular follicular rash on the head and neck. Histology showed dilated hair follicles with orthokeratotic hyperkeratosis and the presence of a mild polymorphous infiltrate, as well as squamous metaplasia of the eccrine ducts.
It is interesting to note that in 1999 Haycox et al.42 described the case of an immunosuppressed patient with progressive alopecia of the eyebrows and erythematous facial papules, producing a leonine facies appearance, and projections of spiny appearance with a follicular distribution. On examination of the skin biopsy by electron microscopy, the authors identified viral particles suggestive of papovavirus. This condition was called trichodysplasia spinulosa and has 3 fundamental features: follicular facial papules that produce a leonine facies, alopecia of the eyebrows and eyelashes, and the characteristic keratotic spines. Cases of trichodysplasia spinulosa have been reported in other states of immunosuppression, such as during chemotherapy for acute lymphocytic leukemia,43 chronic lymphocytic leukemia,44 heart transplant,45 and kidney transplant.46 Recently it has been shown that the virus associated with trichodysplasia spinulosa belongs to the polyomavirus family and the condition was named trichodysplasia spinulosa-associated polyomavirus (TSV).47 From a histological point of view, trichodysplasia spinulosa is characterized by distension and abnormal maturation of the hair follicles, a large number of trichohyaline granules in the inner root sheath cells, and keratin spicules of 1 to 3mm in length emerging from the aberrant follicles. Ultrastructural electron microscopy studies confirmed the presence of viral particles of 40nm in the nuclei of the sheath cells.48
Taking into account the similarities in the clinical presentation between the 2 conditions, such as the appearance of the characteristic keratotic spicules and the presence of alopecia of the scalp and eyebrows, we must consider whether spiny follicular hyperkeratosis is related to or is the same as TSV.
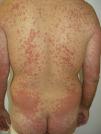
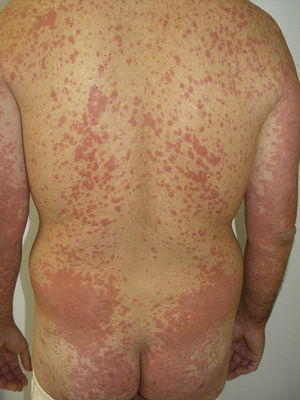
Other Adverse EffectsOther adverse effects have been reported during treatment with sorafenib: 1 case of yellowish skin discoloration (in contrast to sunitinib, with which this adverse effect is much more common) that resolved after interrupting the treatment49; painful erosive lesions50; hemorrhagic erythema marginatum, consisting of confluent, desquamating, slightly atrophic, annular erythematous macules with a marked hemorrhagic border, whose appearance has been related to a good tumor response to the treatment51; atypical toxic dermatitis with a linear morphology52; psoriasiform rashes53–55; diffuse keratotic pilaris-like rash; stomatitis; rashes (Fig. 5); inflamed seborrheic keratosis; cysts15; and erythema multiforme.56
Cutaneous Side Effects of SunitinibSunitinib shares some cutaneous side effects with sorafenib, such as hand-foot skin reaction and seborrheic dermatitis-like rash, but presents other characteristic cutaneous side effects such as yellowish skin discoloration, hair depigmentation, and genital lesions.
Hand-Foot Skin ReactionHFSR occurs in 20% of patients treated with sunitinib and is the adverse skin reaction that most frequently leads to the abrupt withdrawal of treatment for skin toxicity.57 In 1 case, HFSR lesions were reported to have been induced by handling a Blackberry device.58 The clinical characteristics and treatment options of sunitinib-associated HSFR lesions59 are similar to those described in the section on the cutaneous side effects of sorafenib, and they will not therefore be repeated in this section. In a recent study it was shown that skin toxicity occurring during treatment with sunitinib for metastatic renal cell carcinoma was associated with a significant increase in overall and disease-free survival.60 An improvement in psoriasis lesions has been observed in patients who have received treatment with sunitnib61; this would appear logical, as VEGF is related both to the induction of angiogenesis in the dermis and to epidermal hyperplasia, which are both key processes in the pathogenesis of psoriasis.62
Yellowish Skin DiscolorationThis is seen in around 30% of patients treated with sunitinib.57 It usually appears after the first week of treatment and resolves spontaneously when treatment is discontinued. It should be noted that, in contrast to other systemic causes of yellowish skin discoloration, the changes related to sunitinib do not affect the mucosas or sclera. There may be associated yellowish discoloration of the urine, which is due to excretion of the drug via the kidney. This side effect is due to the color of 1 of the metabolites of the drug.9,63
Hair DepigmentationHair depigmentation occurs in around 10% of patients and can affect the scalp hair, eyebrows, and eyelashes.57 It typically develops 5 to 6 weeks after starting treatment with sunitinib and resolves spontaneously 2 to 3 weeks after the interruption of treatment. The hair acquires a characteristic gray color. No histological changes can be seen in the melanocytes of affected follicles.9 There have been cases of hair with bands of normal color and depigmented bands in patients on an intermittent treatment regimen (4 weeks of treatment followed by a 2-week rest). This adverse effect is believed to be due to an inhibition of the c-KIT that modulates the genes of the tyrosinase and tyrosinase-related protein-1 enzymes, which are involved in melanin synthesis.64
Scrotal Skin ChangesBillemont et al.65 reported the presence of cutaneous side effects affecting the inguinoscrotal region in 12% of a series of male patients treated with sunitinib. The authors observed the appearance of erythema and peeling 2 weeks after commencing treatment; the changes resolved spontaneously after withdrawal of the drug. Those authors also described 3 patients with no history of psoriasis who developed severe psoriasiform lesions on the scrotum. The lesions were associated with intense pain and required a reduction of the dose of sunitinib. Recently, lesions with a similar appearance have been reported in the genital region of a woman who received treatment with sunitinib for recurrent and metastatic renal cell carcinoma 7 years after nephrectomy.66 Dose reduction was also required in that patient to achieve symptom control.
Genital changes in the form of erythema of the foreskin, scrotum, and penis, intertrigo, and anal inflammation, were observed in 5 of a series of 8 patients treated with sunitinib. Treatment was interrupted in 4 of the patients due to the effect on quality of life.67
Lesions clinically and histologically compatible with hemangiomas have also been reported in this region; the lesions disappeared on withdrawal of the treatment and recurred with each cycle of administration of sunitinib.68
Recently Chou et al.69 described a patient treated with sunitinib who presented erythema, inflammation, peeling, and the appearance of angiokeratomas on the scrotum, associated with pain. Skin biopsy showed the presence of dilated capillaries with endothelial cells that stained positive for VEGF, but this was not detected in a healthy control group. This finding supports the role of VEGF in the pathogenesis of sunitinib-related genital lesions, as proposed by Billemont et al.65 Although the mechanism is unknown, this hypothesis suggests that VEGF and hypoxia-inducible factor 1-alfa could play an important role. VEGF induces endothelial proliferation, vasodilatation, and increased vascular permeability. Elevated VEGF levels have been reported in the plasma of patients treated with sunitinib, and it has been speculated that there may be a physiological feedback that provokes a reversible increase in circulating and tissue VEGF after the administration of sunitinib (a VEGF receptor antagonist). When VEGF reaches the scrotal region, characterized by a rich blood supply and subject to recurrent friction and trauma, it produces local symptoms such as scrotal erythema, tissue edema, telangiectasias, and the appearance of hemangiomas.65,69 Although Chou et al.69 did not measure circulating VEGF in their patient, they did demonstrate a marked elevation of its levels in scrotal tissue.
Tonini et al.68 proposed 2 possible processes involved in the formation of scrotal hemangiomas: neoangiogenesis (the production of new blood vessels from preexisting capillaries) and vasculogenesis (consisting of the ability of circulating precursor endothelial cells to form new vessels). It has been shown that there is an increase in the number of circulating endothelial cells during treatment with sunitinib, not only of mature cells (circulating endothelial cells [CEC]) but also of their precursors (circulating endothelial progenitors [CEP]). Similarly, increased numbers of CEPs have been found in children with proliferating hemangiomas.68 CEPs have been shown to play a fundamental role in the rapid postnatal growth of infantile hemangiomas.70 The spontaneous disappearance of these lesions after the interruption of treatment with sunitinib may be explained by normalization of CEC and CEP levels.68
Other Adverse EffectsOther cutaneous adverse effects associated with sunitinib have been reported: subungual splinter hemorrhages (in 10%-25% of patients); acneiform facial rash similar to the rash induced by epidermal growth factor (EGF) inhibitors, although very rare and less severe than with EGF inhibitors; scalp dysesthesia similar to that seen with sorafenib; erythematous rash on the trunk; lower-limb edema; alopecia (6%-12%); stomatitis (36%)7; facial edema (25%-50%), which is much less common than the edema caused by imatinib and mainly affects the periorbital region71; eccrine squamous syringometaplasia72; leukocytoclastic vasculitis; pyoderma gangrenosum (reported in 5 cases to date, in all of which the lesions resolved on interruption of the treatment with sunitinib and recurred on its reintroduction)73–77; a case of a rash simulating Gottron papules78; and 4 cases of sunitinib-associated painful skin erosions and ulcers on the lower limbs.79
Cutaneous Side Effects of BevacizumabThe anti-VEGF monoclonal antibody bevacizumab causes less skin toxicity than other similar drugs, but, apart from the typical reactions, this drug has other characteristic cutaneous side effects.
Skin RashAn exfoliative dermatitis or skin rash develops in 19% to 46% of patients receiving treatment with bevacizumab. In contrast to the situation with the EGFR antagonists, the majority of clinical trials found no association between the skin rash and a positive response to treatment. Only 2 cases have been reported in the literature in which the appearance and intensity of the skin rash correlated with a positive response to treatment with bevacizumab, with disappearance of the metastases as the skin lesions progressed. The rash was of erythematous papular lesions and recurred with each new cycle of bevacizumab.80,81 In both cases the lesions resolved spontaneously after completing the treatment with bevacizumab.
Erythematous Papular Rash Associated With the Intravitreous Injection of BevacizumabBevacizumab is used off-label in ophthalmology as an intravitreous injection for the treatment of various diseases related to neovascularization, such as age-related macular degeneration, diabetic retinopathy, and choroidal neovascularization in myopia. There have been 2 case reports of the appearance of erythematous papular lesions on the head and trunk related to the intravitreous injection of bevacizumab for the treatment of choroidal neovascularization.82,83 In one of those cases, the lesions appeared on the head and trunk 6 days after the first injection of bevacizumab, and reappeared 5 days after a second injection, administered 2 months later. In the second case, the patient received 3 injections of bevacizumab; 12 days after the first injection he developed an erythematous papular rash on the forehead and in the temporal regions around the eyes. With topical corticosteroid therapy, the lesions resolved within 8 days but reappeared 14 days after the second injection and 10 days after the third.
Wound HealingAngiogenesis is a part of numerous physiological processes, including wound healing. In animal models it has been shown that bevacizumab inhibits the repair process in the dermis84; this effect is dose-dependent and is reversible if administration of the drug is interrupted. Various effects of bevacizumab on wound healing have been described, such as wound dehiscence, ecchymosis, surgical wound hemorrhage, and an increased risk of infection.85 Scappaticci et al.,84 using the data from 2 clinical trials, investigated complications of surgical wound healing in patients receiving treatment with bevacizumab. They found complications related to delayed healing in 13% of patients undergoing surgery during treatment with bevacizumab, compared with 3% of those not receiving the drug. In contrast, patients who started to receive bevacizumab 28 to 60 days after surgery did not present a higher frequency of complications. It has been shown that would healing is negatively affected not only by the systemic administration of bevacizumab but also by intravitreous administration of the drug.86
Ulceration of StriaeCases of ulceration of corticosteroid-induced striae have been reported in patients with cerebral tumors on treatment with bevacizumab.87 Those authors explained that this phenomenon occurs as a result of the antiangiogenic effect of bevacizumab, which interferes with the repair of tissue previously weakened by corticosteroid therapy.
A case of necrosis of striae has been reported in a patient 1 week after starting treatment with bevacizumab and irinotecan for glioblastoma. Bevacizumab was discontinued 2 months later for lack of efficacy and the lesions healed within a month.88 Recently, a similar case has been described in a child who received bevacizumab and irinotecan for the treatment of recurrent glioblastoma.89 The child developed necrosis of abdominal striae that had appeared secondary to long-term corticosteroid therapy. In the case of cerebral tumors, high-dose corticosteroid therapy is often required for prolonged periods of time to manage cerebral edema. It is therefore particularly important to watch for possible side effects if therapy is started with antiangiogenic agents such as bevacizumab. The literature highlights the importance of the prevention of the appearance of ulcers on striae in these patients and, if they appear, adequate hydration of the area is recommended, together with the use of hydrocolloid dressings.87
Perforating DermatosesThere has been a case report of the appearance of slightly pruritic, umbilicated papular lesions on the neck of a patient who had been receiving bevacizumab for 2 months. The lesions measured 5mm in diameter and had a central keratotic plug. Histology revealed the transepidermal elimination of collagen. Treatment interruption was not required.90
ConclusionsNumerous drugs have been approved in recent years for the treatment of various types of tumor, and many more molecules are in phase 3 or phase 4 clinical trials. The antiangiogenic drugs occupy an important place among these drugs. The antiangiogenic drugs produce skin toxicity in up to 90% of patients. It is therefore very important for the dermatologist to be aware of the cutaneous effects associated with these drugs and to know how to manage them.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
The authors are grateful to Dr Tuneu and Dr López Pestaña of Hospital Donostia, San Sebastian, Spain, for granting them use of their photographic material.
Please cite this article as: Ara M, Pastushenko E. Fármacos antiangiogénicos y piel: efectos cutáneos adversos de sorafenib, sunitinib y bevacizumab. Actas Dermosifiliogr. 2014;105:900–912.