The interaction of high-frequency ultrasound waves with the skin provides the basis for noninvasive, fast, and accessible diagnostic imaging. This tool is increasingly used in skin cancer and inflammatory conditions as well as in cosmetic dermatology. This article reviews the basic principles of skin ultrasound and its applications in the different areas of dermatology.
La ecografía cutánea es una técnica dermatológica de diagnóstico por imagen basada en la interacción de los ultrasonidos de alta frecuencia con la piel. Su carácter no invasivo, rápido y accesible hace que sus aplicaciones en la clínica sean cada vez más amplias, tanto en oncología cutánea como en afección inflamatoria o dermatología estética. En este artículo se revisan los principios de la técnica y las aplicaciones en los distintos ámbitos de la dermatología.
Ultrasound skin imaging is a noninvasive diagnostic technique in which the physical properties of ultrasound are used to examine the skin and skin appendages.1
Both high-frequency (>7MHz) and very-high-frequency ultrasound (>20MHz) can provide a detailed diagnostic analysis of the skin, as they offer sufficient resolution and depth to clearly identify skin structures.2
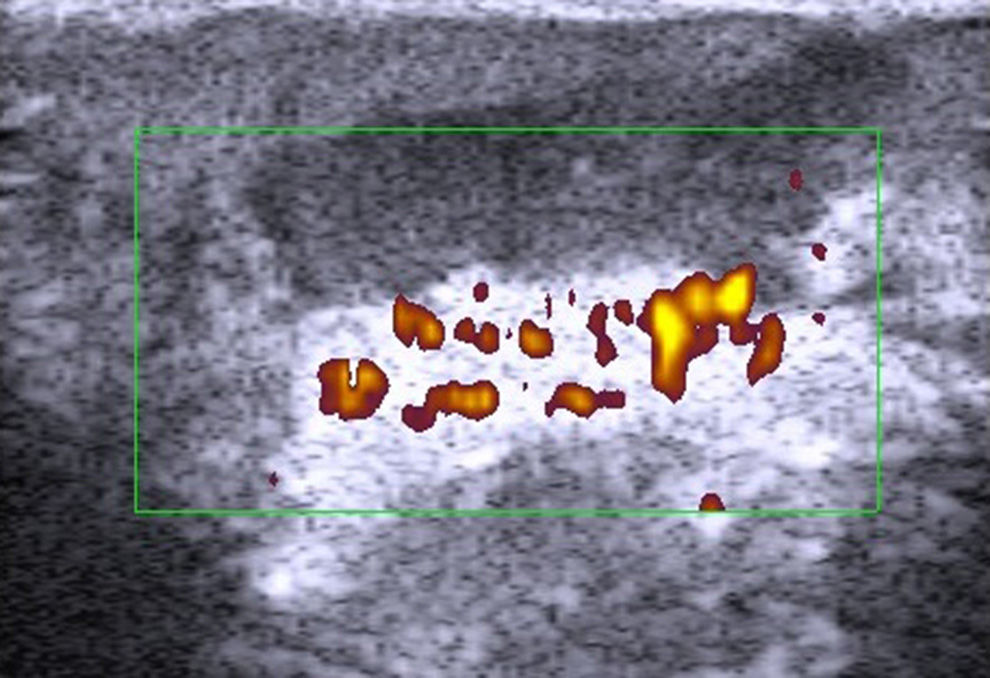
Application of the Doppler effect in ultrasound enables the visualization of physiological and pathological processes involving increased local blood flow, such as inflammation and neoangiogenesis.3
Ultrasound is a relatively new imaging modality in dermatology, and while more studies are needed to support its integration into routine clinical practice, there is evidence of its usefulness in different fields of dermatology.4
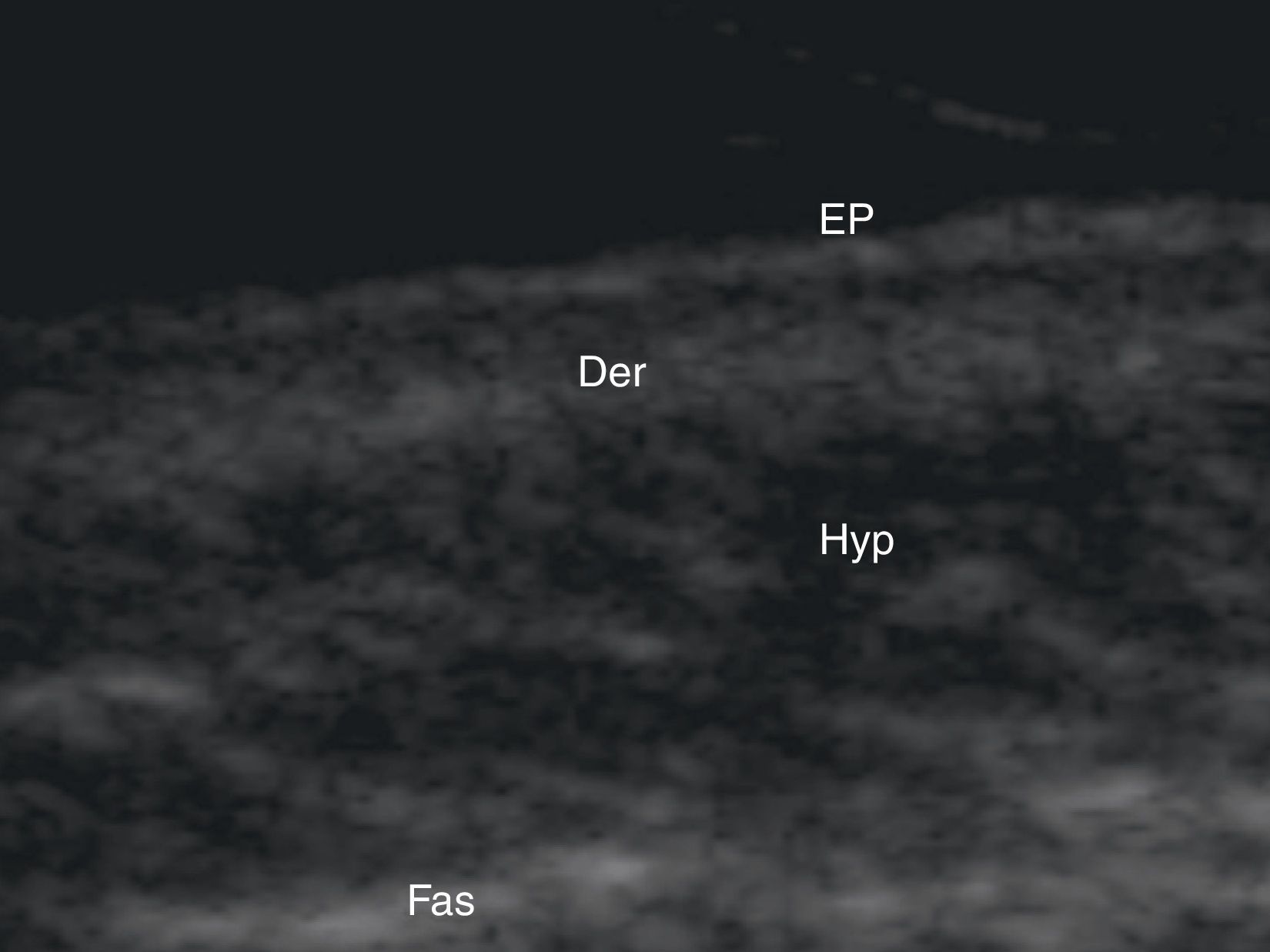
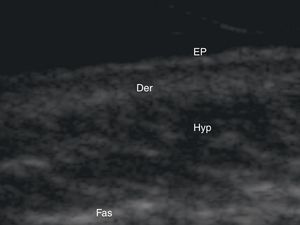
Normal Skin and Skin AppendagesIn B-mode (bright mode) ultrasound imaging, the skin is seen as a series of lines and bands in varying shades of gray, black, and white that correspond to the different layers of the skin5 (Fig. 1).
The epidermis appears as a hyperechoic line, or in certain locations, such as acral sites, as a bilaminar hyperechoic line.6
The dermis also appears as a hyperechoic line but it is not quite as echogenic as the epidermis. A more superficial hypoechoic area, corresponding to the papillary dermis, may also be seen.
Subcutaneous tissue appears under the dermal band as a network of hyperechoic lines that correspond to the septa. Inside are hypoechoic areas that represent the fatty lobules.1
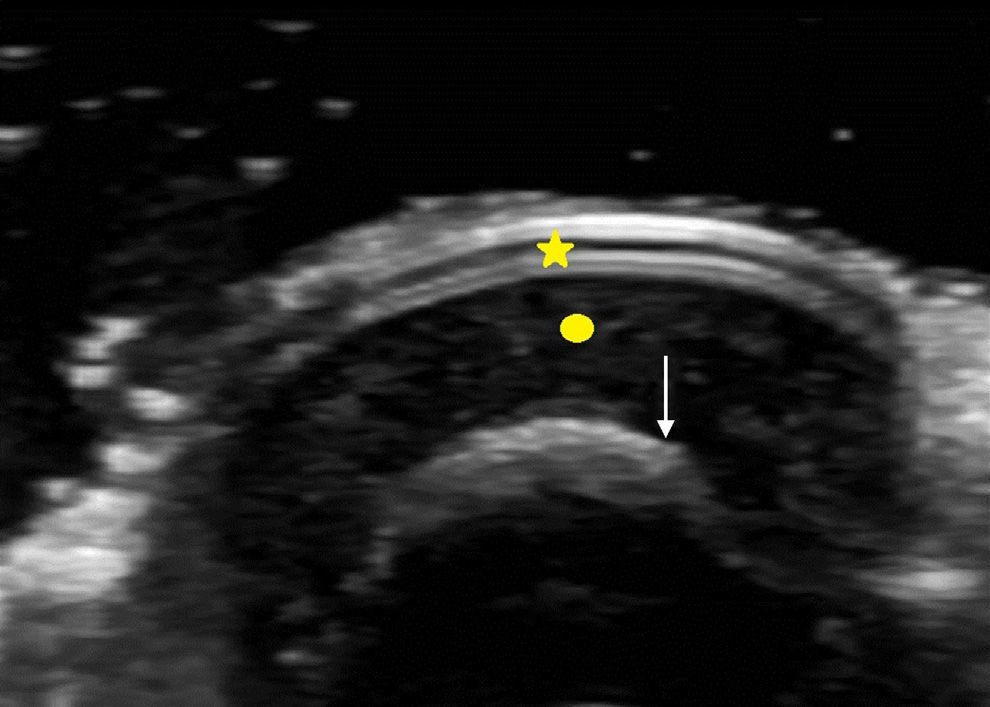
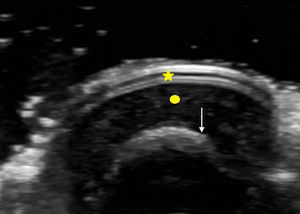
The nail is identified by the presence of a bilaminar hyperechoic structure (the nail plate) above a hypoechoic structure (the nail bed). The nail is closely related to the distal phalanx, which is seen as a continuous hyperechoic line on ultrasound7 (Fig. 2).
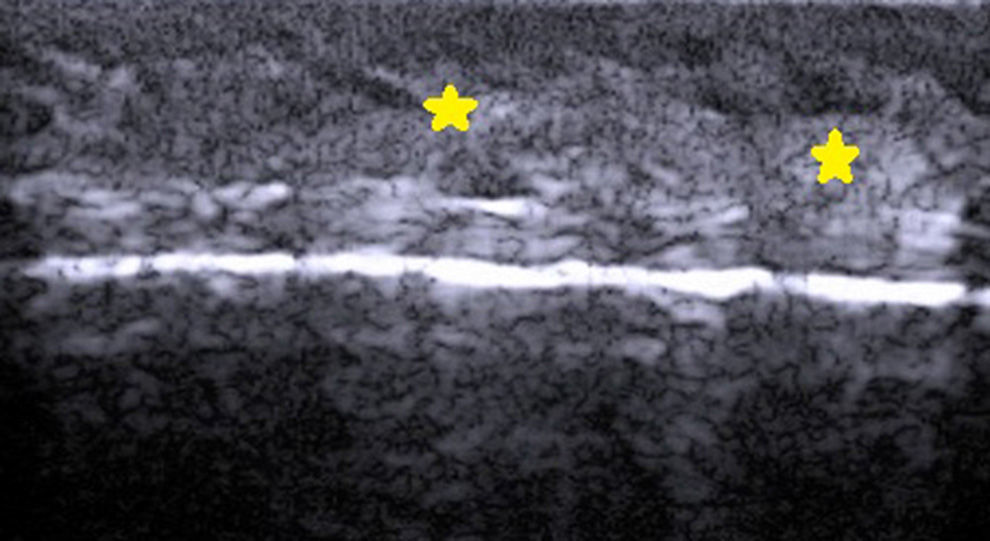
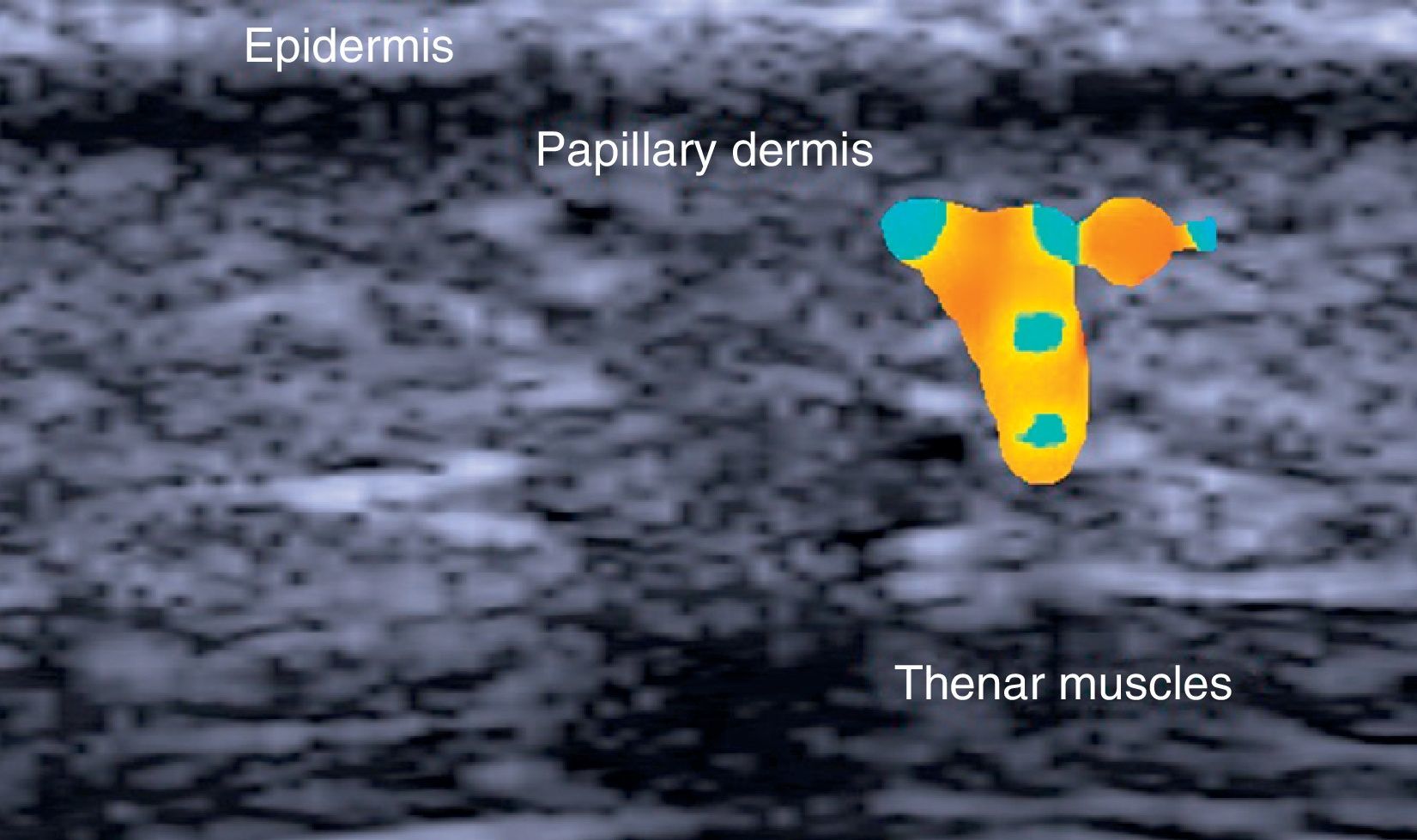
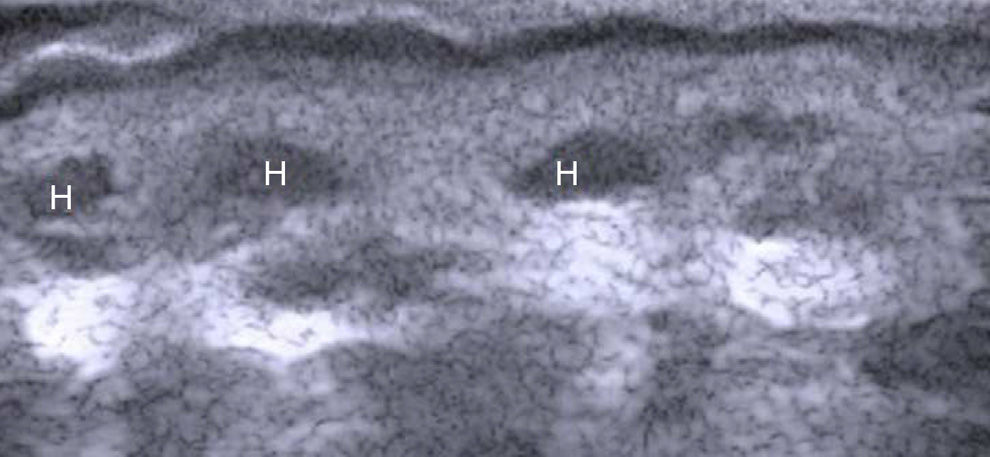
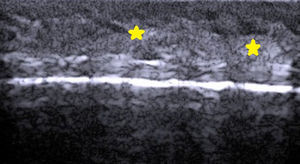
The hair shaft appears as a bilaminar hyperechoic structure, similar to the nail plate, that penetrates the epidermis and dermis at an oblique angle. It is then seen as oblique hypoechoic lines in the dermis and as poorly defined oval hypoechoic areas at the dermal-subcutaneous junction8 (Fig. 3).
Skin CancerBenign Skin TumorsSubcutaneous tumors can occasionally pose a diagnostic challenge. Because ultrasound enables visualization of deep skin structures, it should theoretically offer better diagnostic information than clinical examination only (inspection and palpation).
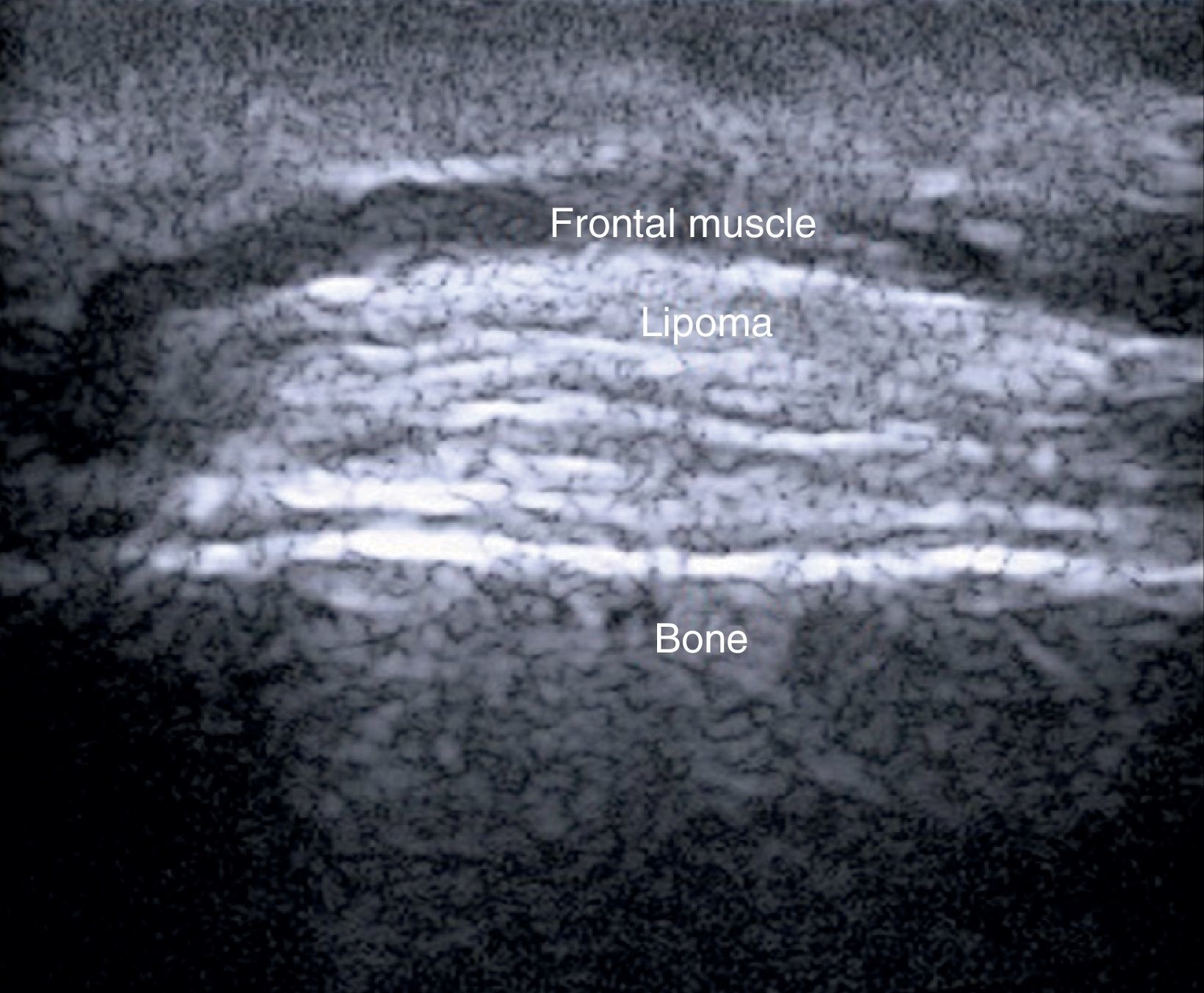
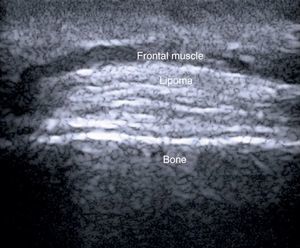
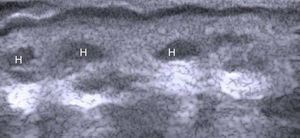
In a study of 183 benign subcutaneous lesions evaluated preoperatively, Kuwano et al.9reported that while palpation aided diagnosis in 64% of lipomas and in 93.5% of epidermal cysts, ultrasound was associated with a diagnostic sensitivity of 88.1% for lipomas (P<.01) (Fig. 4) and 99.3% for epidermal cysts (P<.05).
In a study of 4388 cutaneous lesions by Wortsman et al.,10 the clinical diagnosis was correct in 73% of cases, but when ultrasound was added, diagnostic accuracy increased to 97% (P>.001).
The above findings suggest that ultrasound can improve the preoperative diagnosis of benign subcutaneous lesions.11,12
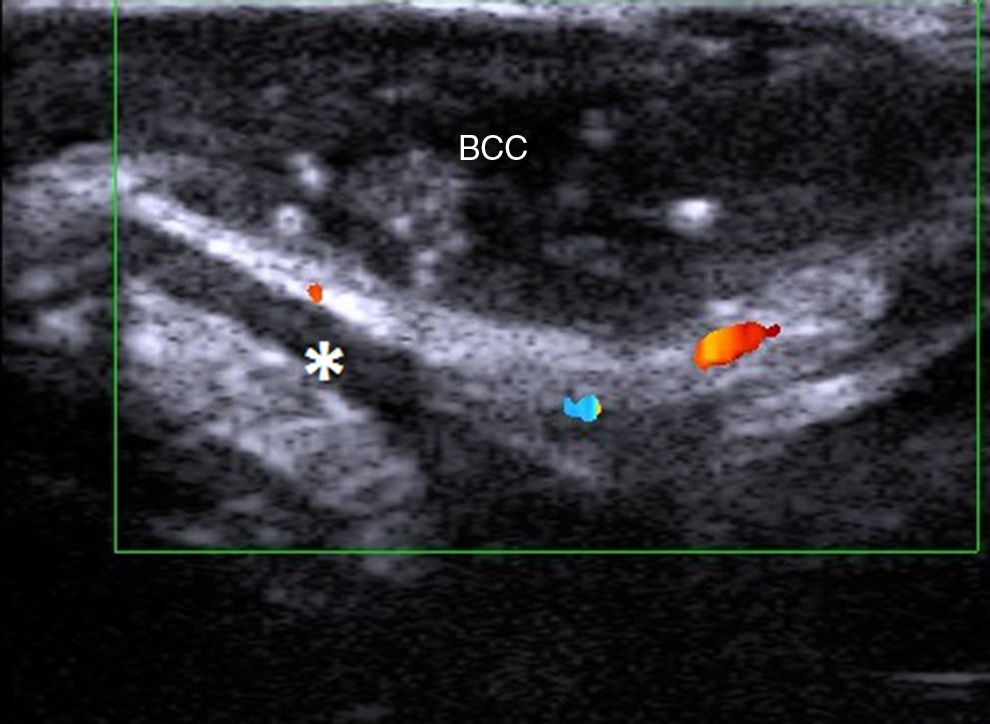
Nonmelanoma Skin CancerLevel of local invasion and infiltration of surrounding structures is important for determining the course of treatment in nonmelanoma skin cancer, basal cell carcinoma (BCC), and squamous cell carcinoma (SCC), and for preventing damage to healthy adjacent structures.
The correlation between tumor thickness determined by ultrasound and histologic depth of invasion has been analyzed in several studies. Crisan et al.,13 for instance, using high-frequency ultrasound (20 MHz), reported a correlation of close to 94%.
Such studies, however, have generated controversy,14 as the gold standard for determining depth of invasion —histology—requires excision and fixation of the tumor specimen, and this may result in shrinkage. Consequently, tumor size determined histometrically will not always coincide with in vivo tumor size.15–18
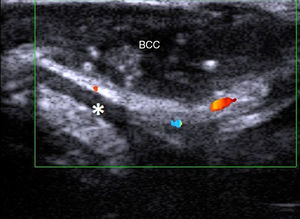
In a study of BCCs located on the face (Fig. 5), a cosmetically sensitive area, Wortman and her group reported a correlation of 0.9 between histologic and ultrasound depth measurements using a 7-15 MHz linear probe.19 The team also analyzed cases in which there was poor correlation and attributed this to overestimation of tumor size on ultrasound due to the presence of sebaceous hyperplasia and peritumoral inflammation. The study also analyzed the presence or absence of cartilage involvement in nasal lesions. Ultrasound ruled out cartilage infiltration in all cases, and these findings were confirmed histologically. In a recent article, Wortsman20 described ultrasound as a first-line imaging modality for the management of facial cutaneous BCC.
Two studies have assessed the value of ultrasound evaluation in Mohs micrographic surgery (MMS). In the first, Marmur et al.21 used 20-MHz ultrasound imaging to assess pre-MMS measurements in 20 patients with BCC and SCC. They found no significant differences between preoperative measurements taken by the ultrasound technician and measurements taken by the surgeon during the first MMS stage, although they did detect satellite BCC lesions by ultrasound that had been overlooked in the clinical examination. In the second study, involving 100 patients with BCC and SCC in different locations, Jambusaria-Pahjlahani et al.22 compared surgical margins delineated by a surgeon prior to MMS, ultrasound margins measured by a trained ultrasound technician, and histologic margins using frozen sections. Based on the histological findings, high-resolution ultrasound predicted disease extent with a sensitivity of 32% and a specificity of 88%. The authors stated that additional refinements were needed before conclusions could be drawn regarding the value of high-resolution ultrasound in assessing disease extent, and also remarked on the need for greater uniformity in study designs and ultrasound criteria.
Tumor depth determined by ultrasound can also be useful for selecting patients for nonsurgical treatment. In a recent study, Smucler et al.23 assigned patients with BCC to nonsurgical treatment with laser, photodynamic therapy (PDT), or laser plus PDT, according to tumor depth as assessed by ultrasound. They achieved a 100% tumor clearance rate in patients with a tumor depth of over 3mm treated with pre-PDT ablative carbon dioxide laser therapy.
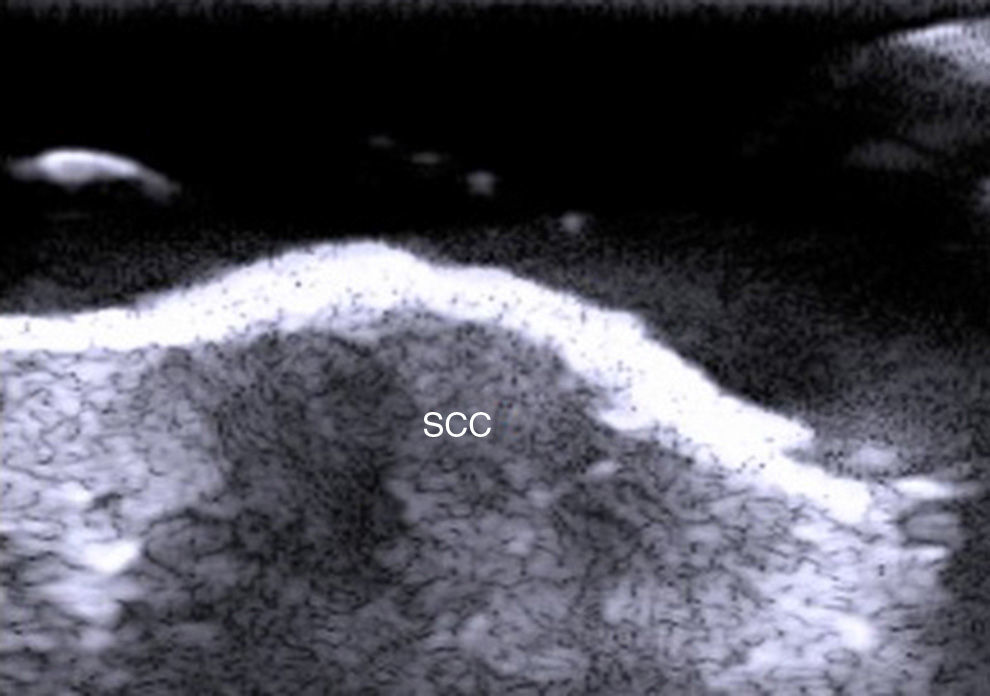
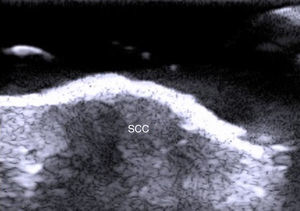
Fewer ultrasound studies have focused solely on SCC, as the keratin produced in this cancer causes an image artifact known as posterior acoustic shadowing, and furthermore, the edges of SCC lesions are more irregular than those in BCC2 (Fig. 6). SCC, and particularly its high-risk variants, can metastasize and recur.24–26
Ultrasound evaluation of lymph nodes can detect regional metastasis in SCC.27 Head and neck SCCs, however, have been found to be located in the vicinity of more aggressive tumors, such as those in the maxillofacial region.27 In the only preliminary study of head and neck SCC, Cousen et al.28 did not find postoperative ultrasound evaluation to be of prognostic value in 47 patients with histologic grade 3 and 4 SCC according to Broders’ classification. Perhaps new ultrasound techniques, such as elastography, will improve detection of very early stages of lymph node metasases.29
MelanomaBreslow depth and the presence of local or distance metastases are prognostic indicators in melanoma and help to guide treatment.30(Fig. 7)
Two key factors influence the correlation between Breslow depth measured by ultrasound and by histology: the mean Breslow depth of the melanomas being studied and the ultrasound equipment used.31 Although numerous studies have been performed, a lack of uniformity in terms of equipment and criteria used to measure Breslow depth makes it difficult to draw clear conclusions.32–34
Researchers at Hospital Costa del Sol in Marbella, Spain recently published a study in which they analyzed 79 cutaneous melanomas using a 15-Mhz ultrasound probe.35 They obtained a mean Breslow depth of 0.8mm, and compared this with mean depth assessed histometrically. They reported moderate correlation for thin melanomas (<1mm) and lower correlation for thicker melanomas. Ultrasound was able to detect stage 1B melanomas with a sensitivity of 82%, a specificity of 80%, a positive predictive power (PPV) of 54%, and a negative predictive power (NPV) of 94%.
In a review of 14 studies analyzing the correlation between melanoma thickness measured by ultrasound and by histology, Machet et al.36 reported correlation rates of around 0.9. Based on the findings of 7 studies with full patient data (869 patients in total), the authors reported that ultrasound-determined margins accurately predicted surgical margins in 72% of cases.
Music et al.,37 in turn, studied 70 melanomas to see whether ultrasound imaging with a probe of 12 to 15 MHz would be capable of distinguishing melanomas thicker than 1mm. They reported sensitivity, specificity, PPV, and NPV values of 92%, 92%, 95%, and 81%, respectively, and concluded that ultrasound was a reliable means of distinguishing between melanomas with a thickness of over 1mm and thinner melanomas.
Ultrasound characteristics of enlarged lymph nodes—length to width ratio <2, hyperechoic center, and absence of hilar vessels—can be used to distinguish between inflammatory nodes and nodes affected by melanoma with a sensitivity of 100% and a specificity of 96%.38
Sentinel lymph node biopsy (SLNB) is a technique used to determine melanoma involvement in the sentinel lymph node, which is the preferential site of metastasis in this disease. The technique involves the injection of radioactive isotopes, followed by surgery to remove the affected node. Preoperative ultrasound has not proven to be superior to SLNB for the identification of melanoma metastasis.39 On comparing ultrasound and SLNB in 623 patients with melanoma, Marone et al.40 showed that ultrasound characteristics were predictive of nodal involvement with a sensitivity of 15%, a specificity of 100%, a PPV of 100%, and an NPV of 87%.
However, in a study of 707 patients with melanoma who underwent preoperative ultrasound assessment, patients with negative lymph nodes on ultrasound had a lower tumor burden and longer overall survival than those with lymph node metastases.41
In a study of 123 patients with melanoma that compared positron emission tomography and ultrasound in the detection of sentinel lymph nodes, ultrasound identified 2 out of 19 positive nodes, while PET detected none.42
Doppler ultrasound of melanomas also has a role to play in the assessment of cutaneous melanomas. Lassau et al.43 showed that detection of neoangiogenesis on ultrasound was predictive of the metastatic potential of melanoma. In a recent study by Srivastava et al.44 of melanomas analyzed by Doppler ultrasound flowmetry (10MHz), neovascularization and higher peak systolic frequency were found to be predictive of survival at 15 years.
The introduction of new ultrasound contrast agents45 and ultrasound elastography46 will probably lead to improved characterization and detection of metastatic lymph nodes. Elastography, and in particular quantification of flow using contrast-enhanced ultrasound, can help to predict response to chemotherapy in patients with advanced melanoma.47
Other Skin TumorsMost of the studies on the use of ultrasound in other skin tumors, such as dermatofibrosarcoma protuberans,48,49 Merkel cell carcinoma,50,51 and cutaneous lymphoma52,53 are mainly descriptive case series.
Inflammatory Skin DisordersUltrasound findings in inflammatory skin disorders include2:
- •
Hypoechogenic areas in the subepidermal portion of the dermis
- •
Increased local blood flow shown by Doppler ultrasound
- •
Hypoechogenic septae when the subcutaneous tissue is affected
- •
Hyperechoic fatty lobules
The above characteristics help to determine the level and extent of inflammation in inflammatory disorders of the skin, hair, and nails.
Infectious DiseasesUltrasound can be used to assess the extent of plantar warts and to monitor treatment response in human papillomavirus infections.54
Ultrasound assessment of abscesses has also become widespread in emergency departments in the United States. According to a report published by a pediatric emergency department in Michigan, ultrasound evaluation led to a change in treatment strategy (drainage vs no drainage) in 15% of cases that had been evaluated by physical examination only.55 An additional advantage of ultrasound in the diagnosis of abscesses is that it can be performed with minimal training.56,57
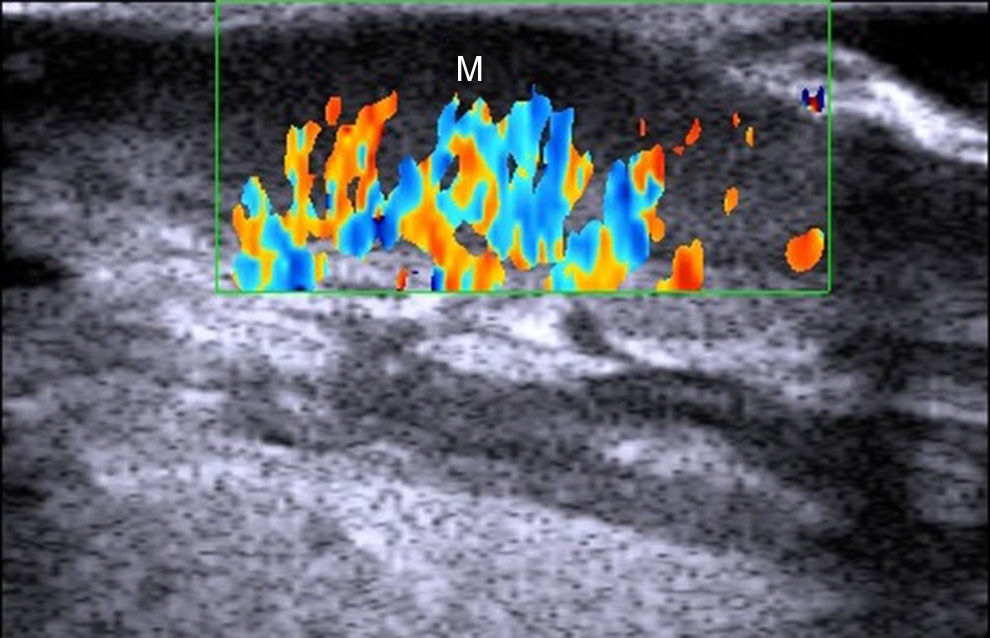
PsoriasisUltrasound characteristics of psoriasis include epidermal and dermal thickening and a subepidermal hypoechoic area with increased blood flow (Fig. 8).58
These ultrasound findings, and in particular, dermal thickness, have been found to be correlated with disease severity measured using the Psoriasis Area Severity Index and other scales assessing the severity or extent of disease.59,60
The effects of topical61 and systemic treatments59,62 for psoriasis can be determined by analyzing changes to the above-described ultrasound characteristics.
In a multicenter study by the Spanish Rheumatology Society, high-frequency ultrasound evaluation showed a reduction in plaque thickness and Doppler signal intensity in the dermis of patients treated with infliximab.62
Nails appear thicker on ultrasound in patients with nail psoriasis than in either healthy patients or patients with atopic dermatitis.63 Nail disease in psoriasis has been correlated with the presence of enthesopathy, even in the absence of clinical signs.63
In an ultrasound study investigating systemic inflammation in psoriasis, researchers at the Universidad de Granada in Spain demonstrated that, compared with controls, patients with psoriasis, and in particular those with nail disease, had lower blood flow in the nails, which was attributed to increased nailflow vessel resistance.64 The authors suggested that psoriasis could be an independent risk factor for microvascular damage.64
Within the concept of psoriasis as an inflammatory systemic disease, ultrasound has been used to evaluate the potential of carotid intima-media thickness as a marker of cardiovascular risk.65,66 In one recent pilot study, intima-media thickness was found to be higher in patients treated with anti-tumor necrosis factor.67 The implications of ultrasound evaluations of systemic inflammation in psoriasis have yet to be clarified.
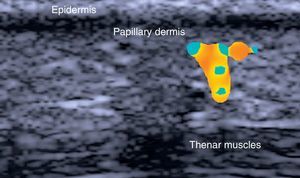
HidradenitisHidradenitis is a predominantly subcutaneous inflammation (Fig. 9) that can be studied in detail by ultrasound.68 On comparing epidermal and dermal thickness in patients with hidradenitis and healthy controls, Wortsman and her group69 found that areas of the body affected by hidradenitis exhibited increased dermal-epidermal thickness and lower echogenicity. Finally, ultrasound can also be used to detect subclinical lesions and occult fistulas in this setting.69
CollagenosisScleroderma is the most widely studied disease by ultrasound, as treatment varies according to the stage of disease (inflammatory or sclerotic).70 In a study of 104 morphea plaques in 59 patients, Wortsman et al.71 showed that ultrasound had a sensitivity of 100% and a specificity of 98.4% for differentiating between the inflammatory and the sclerotic phases. In the same study, ultrasound detected subclinical inflammation in 5 patients with Parry-Romberg syndrome. Attempts have been made to standardize these results to create semi-quantitative scales72 to assess the effectiveness of treatments such as phototherapy.73
Other Inflammatory DisordersIn a recent study comparing features of atopic dermatitis seen by ultrasound and histology, Polanska et al.74 observed a correlation between the thickness of the hypoechogenic subepidermal band and epidermal hyperplasia, parakeratosis, spongiosis, and intensity of inflammatory infiltrates. This band might therefore have potential as a cutaneous inflammation marker. Ultrasound could also be used detect subclinical cutaneous inflammation in normal-appearing skin in patients with atopic dermatitis.75
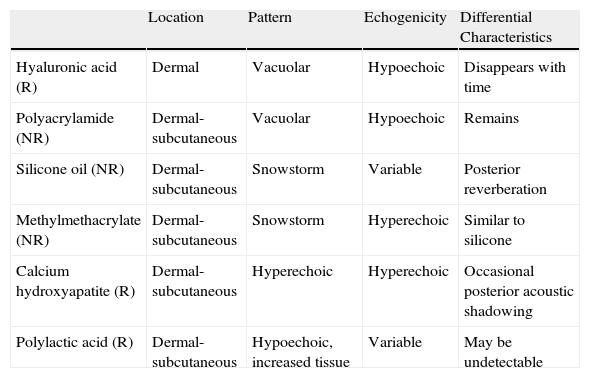
Ultrasound in Cosmetic DermatologyIn recent years, ultrasound has become an important tool for the noninvasive study of cosmetic fillers (Fig. 10) and their potential complications and medico-legal implications.76–78 Dermal fillers have distinguishing ultrasound features (Table 1) that can help to identify the nature of the filler, as well as any changes or complications (e.g., migration, clot formation)79 that can occur postinfiltration. Ultrasound can also be used to assess skin aging through the measurement of the hypoechoic subcutaneous band in sun-exposed areas,80,81 as this reflects the degree of dermal elastosis.
Ultrasound Patterns of the Most Common Dermal Fillers.
| Location | Pattern | Echogenicity | Differential Characteristics | |
| Hyaluronic acid (R) | Dermal | Vacuolar | Hypoechoic | Disappears with time |
| Polyacrylamide (NR) | Dermal-subcutaneous | Vacuolar | Hypoechoic | Remains |
| Silicone oil (NR) | Dermal-subcutaneous | Snowstorm | Variable | Posterior reverberation |
| Methylmethacrylate (NR) | Dermal-subcutaneous | Snowstorm | Hyperechoic | Similar to silicone |
| Calcium hydroxyapatite (R) | Dermal-subcutaneous | Hyperechoic | Hyperechoic | Occasional posterior acoustic shadowing |
| Polylactic acid (R) | Dermal-subcutaneous | Hypoechoic, increased tissue | Variable | May be undetectable |
Abbreviations: NR, nonresorbable; R, resorbable.
Several studies have used subcutaneous band thickness measurement as a quick, noninvasive, and reproducible means of assessing different skin rejuvenation techniques, such as mesotherapy, growth factors,82 and ablative and fractional laser resurfacing.83
The Future of Ultrasound Imaging of the SkinThe future of ultrasound skin imaging is closely linked to the present. On the one hand, dermatologists need to spread word of their experiences with the technique and to continue to learn and experiment, and on the other hand, just as in other areas such as rheumatology, dermatologists performing ultrasound need to standardize terminology and criteria for evaluating skin cancers and inflammatory skin disorders.
The value of recent advances such as elastography and contrast-enhanced ultrasound in dermatology has yet to be determined. The increasing availability of affordable ultrasound equipment specifically designed for dermatologists will also help to foster the use of ultrasound imaging in our field.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We thank Dr Cerezo (President of SEECO), Dr Villegas of Hospital Sanitas Moraleja, and Dr Roustán and Dr Suárez at Hospital Universitario Puerta de Hierro for their support in the early and later stages of cutaneous ultrasound and for their contributions to this article.
Please cite this article as: Alfageme Roldán F. Ecografía cutánea. Actas Dermosifiliogr. 2014;105:891–899.