Tumor necrosis factor receptor–associated periodic syndrome (TRAPS) is a rare autosomal dominant disease included in the group of autoinflammatory syndromes. It is characterized by recurrent episodes of fever and inflammation in different regions of the body. The main clinical manifestations are myalgia, migratory erythematous rash, periorbital edema, and abdominal pain. The diagnosis is reached using gene analysis and prognosis depends on the appearance of amyloidosis secondary to the recurrent episodes of inflammation. Tumor necrosis factor inhibitors and corticosteroids are the most widely used treatments. In recent years, significant advances have been made in the diagnosis and treatment of TRAPS, thanks to a better understanding of its pathogenesis. Dermatologists must be aware that the skin manifestations of TRAPS are particularly important, as they are often diagnostic.
El síndrome periódico asociado al receptor del factor de necrosis tumoral (TRAPS) es una rara enfermedad autosómica dominante que forma parte de los síndromes autoinflamatorios. Se caracteriza por episodios recurrentes de fiebre e inflamación en distintos sitios del organismo, siendo sus principales manifestaciones: las mialgias, el exantema eritematoso migratorio, el edema periorbitario y el dolor abdominal. El diagnóstico se realiza mediante el análisis genético y su pronóstico está determinado por el desarrollo de amiloidosis, secundaria a los episodios inflamatorios repetidos. Los tratamientos más utilizados son los corticoides y los inhibidores del TNF. Durante los últimos años, gracias a un mayor conocimiento de su patogénesis, se han logrado importantes avances en su diagnóstico y tratamiento. Como dermatólogos es importante tener en cuenta que las manifestaciones cutáneas son particularmente importantes en el TRAPS, ya que muchas veces guían al clínico hacia su correcto diagnóstico.
Autoinflammatory syndromes are a group of disorders characterized by recurrent fever, localized inflammation, an absence of autoantibodies, and a tendency toward familial aggregation.1 Autoinflammatory diseases cause several different types of febrile episodes, including fever of unknown origin, periodic fever, and recurrent fever. Differential diagnosis of febrile episodes should therefore include autoinflammatory diseases once infections, malignancies, and autoimmune diseases have been ruled out.2
Cytokines are secreted by macrophages and other cells of the immune system in response to pathogens, against which they mount and inflammatory response. In autoinflammatory diseases cytokines are secreted in the absence of pathogenic stimuli.3
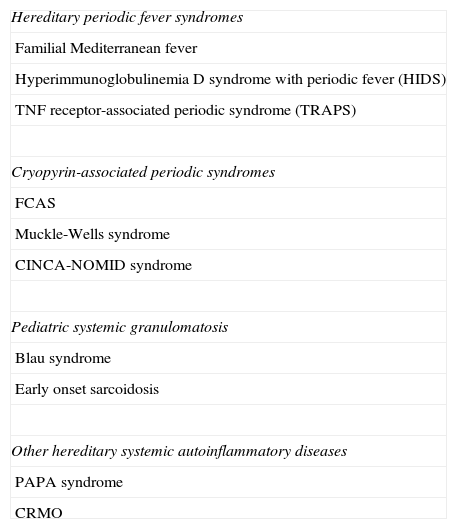
There are several systemic autoinflammatory diseases with a Mendelian pattern of inheritance for which the causative gene has been identified. This group of diseases is divided into several subgroups (Table 1).4
Classification of Hereditary Systemic Autoinflammatory Diseases.
| Hereditary periodic fever syndromes |
| Familial Mediterranean fever |
| Hyperimmunoglobulinemia D syndrome with periodic fever (HIDS) |
| TNF receptor-associated periodic syndrome (TRAPS) |
| Cryopyrin-associated periodic syndromes |
| FCAS |
| Muckle-Wells syndrome |
| CINCA-NOMID syndrome |
| Pediatric systemic granulomatosis |
| Blau syndrome |
| Early onset sarcoidosis |
| Other hereditary systemic autoinflammatory diseases |
| PAPA syndrome |
| CRMO |
Abbreviations: CINCA, chronic infantile neurological, cutaneous, and articular syndrome; CRMO, chronic recurrent multifocal osteomyelitis; FCAS, familial cold-induced autoinflammatory syndrome; NOMID, neonatal-onset multisystemic inflammatory disease; PAPA, pyogenic sterile arthritis, pyoderma gangrenosum and acne syndrome.
Hereditary periodic fever syndromes constitute the most important subgroup of systemic autoinflammatory diseases. This subgroup comprises 2 diseases with a recessive pattern of inheritance (familial Mediterranean fever [FMF], and hyperimmunoglobulinemia D and periodic fever syndrome [HIDS]) and 1 dominantly-inherited condition (tumor necrosis factor [TNF] receptor-associated periodic syndrome [TRAPS]).
In recent years there has been an exponential increase in interest in autoinflammatory diseases by doctors of different specialties, and in the study of these diseases.
In this review we summarize and discuss new data relating to TRAPS that has been published in the last 5 years. To gather this data, we searched the PubMed database using the search term TRAPS. We retrieved 8221 articles, some of which did not pertain to TRAPS. The search term TRAPS TNF receptor returned 179 articles. We selected articles that were published within the last 5 years (78 articles in total). We also reviewed the most important references cited in the selected articles.
Clinical ManifestationsA 1982 study described members of a family of Scandinavian origin who presented episodes of prolonged fever, abdominal pain, myalgia, erythema, conjunctivitis, and/or periorbital edema.5 This condition was termed Hibernian fever, as distinct from Mediterranean fever.
In 1999, 6 missense mutations (i.e., mutations that result in amino acid changes) were discovered in the gene encoding tumor necrosis factor receptor 1 (TNFR1), located on chromosome 12p, in individuals affected by this condition.6 Following this discovery the acronym TRAPS (TNF-receptor-associated periodic syndrome) was coined.
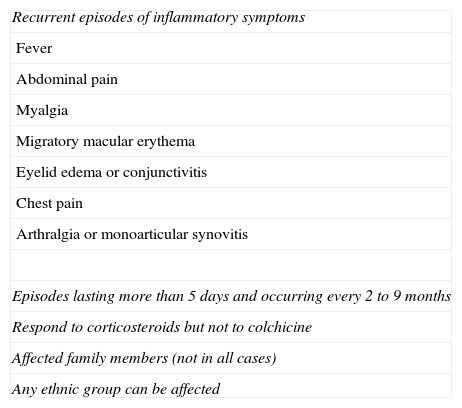
Although the first cases were described in Irish and Central European populations, TRAPS has been reported in countries throughout the world, including Spain.7 The sex distribution is approximately 1:1. The main clinical characteristics of TRAPS are summarized in Table 2.1
Diagnostic Criteria for Tumor Necrosis Factor Receptor-Associated Periodic Syndrome.
| Recurrent episodes of inflammatory symptoms |
| Fever |
| Abdominal pain |
| Myalgia |
| Migratory macular erythema |
| Eyelid edema or conjunctivitis |
| Chest pain |
| Arthralgia or monoarticular synovitis |
| Episodes lasting more than 5 days and occurring every 2 to 9 months |
| Respond to corticosteroids but not to colchicine |
| Affected family members (not in all cases) |
| Any ethnic group can be affected |
It is not necessary for patients to meet all criteria nor is there a minimum number of criteria required to suspect this syndrome.
The symptoms of TRAPS generally appear around 3 years of age (preschool age). Attacks are usually prolonged (lasting up to 3 weeks) and recur with variable frequency.
However, some patients present persistent symptoms that increase and decrease in intensity without clear asymptomatic intervals. Some patients have reported that certain factors or situations can trigger the episodes (physical or psychological stress, ovulation, or menstruation) and in some cases patients experience prodromal symptoms (periorbital edema, general malaise, headache).8
Patients have described prodromal symptoms such as deep muscle pain that increases progressively to reach maximum intensity after 2 to 3 days and subsequently subsides.
Fever is always present in children but may be absent in adults. The most notable differentiating symptom of TRAPS is myalgia, which normally affects only 1 region of the body and varies in intensity throughout the duration of the attack.9
Myalgia is caused by chronic inflammatory fasciitis, not myositis, and thus serum concentrations of creatine and aldolase are usually normal.10
In addition to myalgia, patients usually present a migratory erythematous rash (centrifugal spread), which is frequently maculopapular. This rash often takes the form of a bullseye as the inflammatory episode evolves.
Joint pain is very common, while arthritis, whether monoarticular and oligoarticular, occurs less frequently.8
Ocular manifestations are very common (over 80% of cases) and include conjunctivitis, edema, and/or periorbital pain (unilateral or bilateral). Periorbital edema can occur as a prodromal symptom.
Digestive symptoms are present in a large proportion (up to 92%) of patients with TRAPS and often manifest as abdominal pain, which may be secondary to inflammatory peritonitis or inflammation of the muscles of the abdominal wall.4,8
Vomiting and constipation also occur in many patients, some of whom require surgical intervention for acute abdomen, usually caused by sterile inflammatory peritonitis.
Other less common manifestations include lymphadenopathy, chest pain (with pleural or intercostal muscle involvement), and testicular pain and swelling.
Many patients with TRAPS report that they do not feel completely well between episodes. Quillinan and coworkers11 demonstrated that subclinical fasciitis and arthritis persist between febrile attacks.
The most serious complication of TRAPS is the development of secondary amyloidosis due to recurrent inflammatory processes over several years. TRAPS patients with mutations that affect cysteine amino acids have a higher risk of developing amyloidosis than those carrying noncysteine mutations (24% versus 2%, respectively). The kidney is usually the first organ affected by amyloidosis, and proteinuria is the earliest indicator.8,12
PathogenesisStudies of the pathogenesis of TRAPS have demonstrated defective TNF-dependent cellular signaling, an unexpected finding given the hyperinflammatory phenotype of the disease. Several studies have found that the majority of mutated TNF receptors never reach the cell surface and remain trapped in the endoplasmic reticulum, where they elicit an intracellular inflammatory response resulting in the constitutive expression of proinflammatory cytokines.13,14
Elevated levels of IL-22 expression have been reported in a patient with TRAPS. This T cell-derived cytokine induces inflammation of the liver, pancreas, intestine, and skin. Elevated IL-22 levels have also been described in patients with psoriasis or pityriasis rosea.15
DiagnosisWith the exception of genetic analysis, there are no specific laboratory tests for TRAPS. During episodes an intense acute reaction occurs, involving neutrophilia, left shift, thrombocytosis, anemia, and increases in the erythrocyte sedimentation rate and in the levels of C-reactive and serum amyloid proteins. A polyclonal increase in immunoglobulins may occur, and no autoantibodies are detected. These alterations tend to attenuate during asymptomatic periods.1,8
Although TRAPS usually begins in early childhood, this disease has also been diagnosed in adult patients. It is thus inadvisable to rule out the presence of this syndrome based solely on the age of patient.16
Eighty-six TNFRSF1A mutations (109 sequence variants) able to induce the development of this disease have been described.3
The relationship between mutations and the disease phenotype is unclear, due to the large degree of heterogeneity between different mutations and between patients with the same mutation.17
However, certain mutations are associated with milder forms of the disease. For example, the R92Q substitution generally causes a much milder disease course than that seen in patients with a structural mutation in the TNFRSF1A gene.18,19
It is likely that many TNFRSF1A mutations have yet to be described. Many patients display all the clinical characteristics of TRAPS but carry none of the associated mutations described to date.20,21
A recent review summarized all skin manifestations in TRAPS,22 which included migratory plaques, rashes, urticaria, erysipelas-like erythema, edematous plaques, periorbital edema, and/or conjunctivitis. Skin lesions were usually described as painful due to underlying myalgia, and were histologically nonspecific. The most characteristic finding was a dermal infiltrate of lymphocytes and monocytes. Immunohistochemistry revealed that the infiltrate consisted of CD3+, CD4+, CD8+, CD68+, CD79a−, and CD20− cells. None of the biopsies indicated leukocytoclastic or granulomatous vasculitis, or multinucleated macrophages.
It is clear that patients who present the main symptoms of this syndrome should undergo genetic testing. However, problems arise with patients who present only some of the possible symptoms of TRAPS. For example, cases of TRAPS patients whose only manifestation is recurrent pericarditis have been reported.23
A study of 131 patients with acute recurrent pericarditis found that TRAPS was the cause of this condition in only 6% of the cases studied.24 The authors concluded that patients with recurrent acute pericarditis in whom TRAPS is suspected (and hence who require genetic testing) are those with a family history of pericarditis or periodic fever syndromes, a poor response to colchicine, and who experience recurrences within 1 year of diagnosis or during colchicine treatment,25 as well as patients who require immunosuppressive agents to control the disease.
A V20A mutation that causes regular gastrointestinal symptoms (nausea, vomiting, diarrhea, and extreme fatigue) without other manifestations of TRAPS (fever, arthralgia, or skin lesions) has also been described.26
Association of TRAPS With Other DiseasesTNF signaling is implicated in the pathophysiology of multiple sclerosis. The R92Q mutation in the TNFR1 gene is a genetic risk factor for the development of multiple sclerosis. Cases of multiple sclerosis patients who carry the R92Q mutation and also present symptoms of TRAPS have been reported. This mutation appears to be a genetic risk factor for both diseases. An alteration in TNF-TNFR signaling could induce an increase in proinflammatory signaling, while the final clinical phenotype may be determined by other as-yet-unknown genetic or environmental factors.27–29
A previously unknown TNFR1 mutation that causes symptoms of TRAPS and HIDS was recently discovered in a child.30
The case of a patient carrying a mutation in the TRAPS gene and another in the gene that causes FMF has been reported; the patient's phenotype was a mixture of the 2 pathological processes.31
TreatmentThe complexity of the pathogenesis of TRAPS is evidenced by the number of different treatments that have been used in this condition, with varying degrees of success.17
Colchicine can potentiate the effect of corticosteroids in controlling attacks, and its use during asymptomatic periods can decrease the risk of amyloidosis. Nonsteroidal anti-inflammatory drugs (NSAIDs) are used to attenuate symptoms.8,32
Corticosteroids at doses of over 20mg/d can provide short-term control of TRAPS. However, coadministration of NSAIDS may be required due to the development of corticosteroid tolerance and dependence over time.33
Etanercept, administered subcutaneously at a dose of 25mg twice a week, has been shown to reduce both the duration and frequency of attacks, allowing a reduction in corticosteroid dose.34–36 However, the benefits of etanercept treatment are variable and in some cases are not sustained.37 This treatment has been shown to reverse nephrotic syndrome induced by amyloidosis in a patient with TRAPS, but failed to decrease the amount of amyloid deposited.38
Patients with TRAPS should not be treated with the anti-TNF monoclonal antibody infliximab, as this can induce paradoxical inflammatory reactions.39,40 Adalimumab also appears to induce paradoxical inflammatory reactions.37 However, some patients with TRAPS respond well to infliximab treatment.41 It is postulated that the response to infliximab and the development of paradoxical inflammatory reactions may depend on the type of mutation carried by the patient.
Anakinra, a recombinant antagonist of the IL-1 receptor, has shown promising results in the treatment of TRAPS,42–44 particularly in patients who do not respond to etanercept.45 However, a more recent study demonstrated a lack of response to anakinra by TRAPS patients carrying the T50M mutation (who had previously failed to respond to etanercept).46
Treatment of a TRAPS patient with tocilizumab, a humanized monoclonal antibody that binds to the IL-6 cellular receptor, was found to abort an acute episode and prevent further episodes, although levels of IL-1a and IL8 remained elevated in this patient.47
An improvement was reported in a patient with TRAPS who was treated with moxifloxacin, although this effect was not observed in 3 other patients who received the same treatment.37 It is not known whether the beneficial effects of moxifloxacin were due to the elimination of bacterial antigens or its anti-inflammatory action.48
ConclusionsGiven the risk of amyloidosis it is essential to diagnose patients with TRAPS.
Dermatologists should be familiar with this syndrome, as a large proportion of patients can be diagnosed by their skin symptoms.
Ethical ResponsibilitiesProtection of persons and animalsThe authors declare that no experiments were performed on humans or animals during the course of this study.
Data confidentialityThe authors declare that they have followed the protocols of their place of work pertaining to the publication of patient data and that all patients included in the study were appropriately informed and provided written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of InterestThe authors declare that they have no conflict of interest.
Please cite this article as: Aguado-Gil L, et al. Novedades en el diagnóstico y tratamiento del síndrome periódico asociado al receptor del factor de necrosis tumoral. Actas Dermosifiliogr. 2013;104:617–22.