Phototherapy, classic systemic treatments (methotrexate, acitretin, and ciclosporin), and biologic agents (etanercept, infliximab, adalimumab, and ustekinumab) constitute a broad therapeutic arsenal that increases the likelihood of achieving control of severe and extensive disease in patients with psoriasis. Acitretin continues to be a very valuable tool in both monotherapy, in which it is combined with other systemic treatments (classic or biologic), and in sequential therapy. Thanks to its lack of a direct immunosuppressive effect and its ability to achieve a long-term response, acitretin has an important role in the treatment of psoriasis, although this has not always been acknowledged in relevant treatment guidelines.
We present consensus guidelines for the use of acitretin in psoriasis drawn up by the Psoriasis Group of the Spanish Academy of Dermatology and Venereology. These guidelines provide a detailed account of acitretin, including pharmacological properties, indications and contraindications, adverse effects, and factors that should be taken into account to enhance the safe use of this drug. They also propose treatment strategies for use in routine clinical practice. The overall aim of these guidelines is to define the criteria for the use and management of acetretin in psoriasis.
La fototerapia y los tratamientos sistémicos clásicos (metotrexato, acitetrina, ciclosporina), junto con las denominadas terapias biológicas (etanercept, infliximab, adalimumab, ustekinumab), permiten al dermatólogo disponer de un arsenal terapéutico amplio que aumenta las posibilidades de control de pacientes con psoriasis grave y/o extensa. La acitretina sigue siendo de gran utilidad tanto en monoterapia como combinada con otros fármacos sistémicos (clásicos o «biológicos»), o en terapia secuencial. Se distingue por no ser inmunosupresor directo y mantener respuestas a muy largo plazo, lo que le confiere un papel relevante en el tratamiento de la psoriasis, que no siempre ha sido reconocido en las diversas guías terapéuticas de esta enfermedad.
Se presenta una guía de uso de acitretina consensuada por los miembros del Grupo de Psoriasis de la Academia Española de Dermatología y Venereología, en la que se exponen de forma detallada aspectos de la farmacología del fármaco, sus indicaciones y contraindicaciones, su eficacia antipsoriásica, los efectos adversos asociados al fármaco, las acciones a tener en cuenta para aumentar la seguridad de su uso, y se propone diversas estrategias terapéuticas de aplicación en la práctica clínica habitual. El objetivo global es facilitar los criterios de indicación y manejo de la acitretina en pacientes con psoriasis.
The treatment of psoriasis has changed considerably in the last 10 years with the advent of biologic therapy—a development that has increased the arsenal of drugs available for the systemic treatment of this condition. Acitretin fulfills a unique role in the strategies used to treat psoriasis because its mechanism of action is different from that of other systemic drugs. This distinction is the main reason for the continued usefulness of acitretin in the treatment of psoriasis despite the more than 35 years that have elapsed since it was first synthesized and the 25 years since it was first introduced in Spain. Acitretin continues to be useful as monotherapy and in combination with other systemic treatments or phototherapy, and its role as a rescue drug or in combination with biologic agents is particularly interesting, as has been shown by recent reviews on this topic.1–5
The retinoids are a group of nonsteroid hormone compounds related to retinol. These intracrine and paracrine mediators intervene in a wide range of essential biologic processes in vertebrates, including embryogenesis, morphogenesis, and organogenesis, the regulation of cell growth, differentiation, and apoptosis, as well as immune regulation.6–8 Retinoids exert their effects by binding to cytoplasmic proteins, which determine their intracellular distribution, and by binding to and/or activating nuclear receptors belonging to the superfamily of the ligand-inducible transcription factors, including steroid, vitamin D3, and thyroid hormone receptors. There are 2 known families of retinoid nuclear receptors, the retinoic acid receptor (RAR) and the retinoid X receptor (RXR), each one comprising 3 types and various isoforms. In normal skin, the concentration of RXR is 5 times that of RAR. Before binding to the nuclear response elements, which determine the transcription of the various retinoid-dependent genes, the activated retinoid receptors bind to form homodimers (RAR/RAR, RXR/RXR) or heterodimers (RAR/RXR, RAR/VDR or RXR/VDR); the target genes sensitive to therapeutic retinoids respond predominantly to the RXR/RAR heterodimers.9 The development of the retinoids currently on the market has mirrored the discovery of the specificity of action of different intranuclear receptors in an effort to reduce the toxicity and increase the specificity of action of these drugs.8,10–12
The retinoids were first synthesized in the 1970s to assess their usefulness in skin cancer,13 and their use in dermatology was soon extended to the treatment of other proliferative diseases.14 In psoriasis, their usefulness arises from their effects on keratinization, cell proliferation and inflammation,12,15–18 and immune regulation.19,20
When first developed in 1972, the monoaromatic retinoid etretinate had a considerable impact on the systemic treatment of psoriasis and other disorders of keratinization because of its antiproliferative activity and effect on cell differentiation, especially in epithelial tissues.9,21,22
The introduction of etretinate, the first retinoid used to treat psoriasis, revolutionized psoriasis therapy, adding an interesting new option to the limited therapeutic arsenal available at that time for severe cases—essentially methotrexate and psoralen-UV-A (PUVA) therapy. The introduction of etretinate was also accompanied by a methodological revolution because the Psoriasis Area and Severity Index (PASI) was first used to assess the efficacy of the new retinoid in clinical trials.23
Like all the synthetic retinoids, etretinate is highly teratogenic, and this risk—further increased by the drug's long elimination half-life—greatly restricted its use in women of reproductive potential. As a result, etretinate was replaced in 1997 by its active metabolite, acitretin, a drug with similar efficacy but a shorter half-life. It was hoped that this shorter half-life would reduce the duration of the drug's teratogenic effects; however, it did not.
Even though acitretin is used to treat many skin conditions, the present review will focus only on its usefulness in psoriasis. We felt it would be of interest to develop guidelines by consensus among the members of the Psoriasis Group of the Spanish Academy of Dermatology and Venereology.
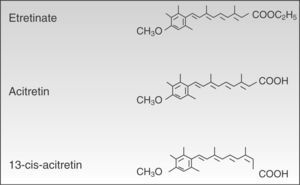
PharmacologyAcitretin (C21H26O3, PM 326.42934 [g/mol]) (Fig. 1) is a second-generation monoaromatic retinoid and a free acid form and active metabolite of its precursor etretinate. It has the advantage over its precursor of having a much shorter half-life.
Despite their structural similarity, the 2 drugs have very different physical and chemical properties. Owing to its better pharmacokinetic profile, efficacy, and safety acitretin has now completely replaced etretinate.
Mechanism of ActionAcitretin acts by modulating the proliferation of epidermal keratinocytes. In hyperproliferative tissue, such as psoriasis plaques, acitretin has an antiproliferative effect, whereas in healthy tissues it induces proliferation.24 In psoriasis, this antiproliferative action reduces desquamation, erythema, and the overall thickness of the lesion. Some authors have also attributed retinoids a role in the modulation of the T-cell response,25 the inhibition of chemotaxis, and the activation of polymorphonuclear leukocytes.26 Unlike other systemic antipsoriatic therapies, acitretin is not considered to be cytotoxic or immunosuppressive.
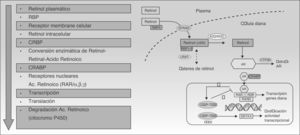
Acitretin binds to RAR and RXR nuclear receptors, activating all 3 subtypes (α, β, and γ) and modulating the transcription of the genes that code for various proteins involved in the pathogenesis of psoriasis.27 Although retinoids act primarily by binding to nuclear receptors, they also act indirectly by antagonizing a number of transcription factors, competing with coactivator proteins which are essential for the activation of genes on which retinoids have no direct effect (Fig. 2).10,28,29 In addition to its direct mechanism of positive transcription, acitretin also exerts an indirect effect by inhibiting certain genes that regulate proliferation, angiogenesis, and inflammation in psoriasis.12,30
Mechanism of action of retinoids. Abbreviations: ADH, alcohol dehydrogenase; COUP-TFII, chicken ovalbumin upstream promoter-transcription factor II; CRABP, cellular retinoic acid-binding proteins; CRBP, cellular retinol-binding proteins; CYP, cytochrome P450, family 26; FOG2, friend of GATA; GATA4, guanine, adenine, thymine, adenine; LRAT, lecithin retinol acyltransferase; RAR, retinoic acid receptors; RARE, retinoic acid response elements; RBP, retinol binding protein; RDH, retinol dehydrogenase, RXR, retinoid X receptors; STRA6, stimulated by retinoic acid 6.
Although the specific action that derives from this acitretin-induced transcription and inhibition is not fully understood, various hypotheses have focused on 2 areas of action: the modification of the metabolism and action of endogenous retinoids,29,31 and the overall immune regulation of the inflammatory molecules and cells involved in psoriasis,19,31,32 particularly helper T (Th) 1 and Th17 cells.18,20,33 These theories would explain the mechanisms of action that make acitretin capable of reversing the pathological and clinical manifestations of plaque psoriasis.18,34–37
Absorption and DistributionThe mean absolute bioavailability of acitretin is 59% (range, 36% to 95%). Absorption is linear up to 50mg/d and nonlinear at higher doses. The rate and extent of absorption can be 2 to 5 times higher when acitretin is taken with food.38,39
Absorbed acitretin is transported bound to plasma proteins (95%)—mostly albumin—with the remainder binding to high-density lipoprotein, low-density lipoprotein, and very low-density lipoprotein.38,40 The drug is distributed rapidly throughout the body and does not accumulate in any particular tissue. It is about 50 times less lipophilic than etretinate, and does not therefore tend to accumulate in adipose tissue.41
MetabolismAcitretin is metabolized primarily by the liver, giving rise to 2 metabolites: 13-cis-acitretin and etretinate. The amount of 13-cis-acitretin formed is independent of dose, formulation, and administration with food. Steady state concentrations of acitretin and 13-cis-acitretin are reached after approximately 3 weeks of daily administration of acitretin.
It has been found that etretinate is naturally produced from acitretin through a re-esterification process that varies in each individual.42 The amount of etretinate produced is very small, but this process is potentiated by the presence of ethanol through the action of a liver enzyme that catalyzes the ethyl esterification of acitretin to etretinate.17,43 Since the accumulation of etretinate in fatty tissue, and in the liver, kidney, brain, and testes, is 50 times greater than that of acitretin, retinoid activity can persist for a long time even after the patient has stopped taking acitretin.
Once it has exerted its action on the target cell, the unused acitretin and etretinate are converted to polar metabolites by the enzymatic action of the cytochrome P450 complex.10,44
Half-lifeChemically, acitretin and etretinate are very similar, with the only difference being that etretinate is the ethylester form of acitretin. This small chemical difference, however, results in major differences in the pharmacokinetic profiles of the 2 drugs. Etretinate, unlike acitretin, is an uncharged molecule and therefore highly lipophilic, a characteristic that leads to considerable accumulation of the drug in body fat. Acitretin, on the other hand, has a negative ionic charge and therefore accumulates less in adipose tissue. Its higher rate of clearance and lower volume of distribution means that it has a much shorter elimination half-life than etretinate (50hours vs 120 days).45–47 In fact, this was the main reason why acitretin replaced etretinate in clinical practice.
However, the hydrolysis that occurs naturally in the human body to convert etretinate to acitretin can be reversed in the presence of heavy alcohol consumption.46 Etretinate has been detected in the plasma of patients on monotherapy with acitretin, and since etretinate accumulates in adipose tissue and has a long half-life, its presence prolongs the teratogenic potential of acitretin.
Larsen et al46 reported that maximum plasma concentrations of acitretin were reached between 0.9 and 4.6hours after oral administration, with an absorption half-life ranging from 0.2 to 1.7hours and a distribution half-life of between 1.2 and 3.5hours.46 In the same study, the elimination half-life of the drug after cessation of treatment varied between 16.5 and 111.1hours (mean [SD], 47.1hours [29.8]), while that of its metabolite (13-cis-acitretin) varied between 36.5 and 249.4hours (mean [SD], 119.4 [7.4]).
ExcretionThe conjugates produced by the metabolism of acitretin and its metabolite 13-cis-acitretin are eliminated by the kidney (16% to 53%) or excreted into bile and ultimately eliminated in feces (34% to 54%). Acitretin is eliminated within approximately 49hours (range, 33 to 96hours), its metabolite, 13-cis-acitretin within 63hours (range, 28 to 157hours), and etretinate within approximately 120 days (maximum up to 168 days).
Trace amounts of acitretin are excreted in human milk (30–40ng/mL),48 and it is estimated that the infant would ingest only 1.5% of the maternal dose. Traces of the drug have also been found in seminal fluid at very low concentrations (12.5ng/mL).
PharmacogeneticsTo date investigators have identified more than 20 genes that regulate the metabolism of retinoids, 6 genes that encode the nuclear receptors involved in signal transduction, and many others that code for regulators of this pathway.49 Further study and greater understanding of these genetic variations and isoforms will lead to a better understanding of the specific mechanism of action of the retinoids in general, and acitretin in particular.
IndicationsThe summary of product characteristics (SPC) for Neotigason specifies the following indications: severe extensive psoriasis that is resistant to other forms of therapy, palmoplantar pustular psoriasis, severe congenital ichthyosis, and severe Darier disease. Thanks to its to its lack of a direct immunosuppressive effect and its ability to achieve a very long-term response with continued therapy, and in spite of the slowness of clinical response, acitretin continues to have an important role in the treatment of psoriasis. Because of its ability to regulate keratinization, acitretin should be considered in the forms of psoriasis in which the desquamative component predominates over the inflammatory or infiltrative component.
Acitretin is a first-line option in pustular psoriasis,50–52 especially the palmoplantar form,53 and as a component of long-term treatment strategies involving combination, rotation, or sequential therapy.
Although acitretin has traditionally been considered a first-line treatment for psoriatic erythroderma, it should be noted that this indication was established by comparison with etretinate54,55 under the assumption that the proven equivalence between etretinate and acitretin in the treatment of psoriasis vulgaris would also largely hold true for erythroderma.45,56 The use of acitretin in the treatment of erythroderma has not been studied directly and evidence demonstrating its value in this setting is scant.36 In our opinion, now that other faster acting and more effective first-line drugs are available (such as ciclosporin, for example), acitretin should no longer be considered a first-line option in psoriatic erythroderma.
Because its mechanism of action does not involve immunosuppression, acitretin is recommended as a first-line systemic treatment in patients with melanoma, solid tumors, or lymphoproliferative malignancies, and in patients with human immunodeficiency virus infection.57,58 Acitretin is not indicated in the treatment of psoriatic arthritis.53
ContraindicationsAcitretin is absolutely contraindicated in pregnancy and in patients who are allergic to the product or any of its components. It is relatively contraindicated in women of reproductive potential, women who are breastfeeding, patients with hepatic or renal impairment, patients with poorly controlled dyslipidemia, and patients receiving concomitant tetracyclines; in these groups of patients the risk-benefit ratio must be carefully assessed. Table 1 summarizes the contraindications to acitretin treatment.
Contraindications to the Use of Acitretin.
| Relative contraindications |
| Mild hepatic impairment (adjust dose) |
| Mild renal impairment (adjust dose) |
| Alcohol consumption (liver toxicity, re-esterification to etretinate) |
| Drug interactions (increases toxicity) |
| Concomitant organ toxic medication (increases toxicity) |
| Active infections (assess the possibility of acitretin toxicity exacerbating infection) |
| Poorly controlled dyslipidemia |
| Metabolic syndrome |
| Uncooperative or noncompliant patient |
| Pediatric or elderly patient (lower tolerance for toxicity?) |
| Absolute contraindications |
| Pregnancy (contraception starting 1 month before treatment and the patient must wait 2 years after cessation to become pregnant) |
| Severe liver failure |
| Severe kidney failure |
| Allergy to drug components |
The commercial preparation of acitretin (Neotigason) is available in 2 presentations: 10mg and 25mg capsules in boxes of 30 capsules.
The inactive excipients are glucose, sodium ascorbate, cellulose, iron oxide black (E172), iron oxide yellow (E 172), iron oxide red (E 172), titanium dioxide (E 171), and gelatin.
PosologyAcitretin is administered orally, preferably with food (to enhance absorption). The dose is not weight adjusted. Etretinate dosage was weight adjusted, which is perhaps why this information still appears in some acitretin guidelines.59 The optimal therapeutic dose in monotherapy is between 25 and 50mg/d.60,61 It can be administered in a single or twice-daily dose. Higher-doses (50–75mg/d) are more effective, but the appearance of adverse effects usually makes it necessary to reduce the dose or discontinue treatment.61–63 In fact, no clinical trials have been carried out with doses higher than 75mg/d. Doses under 25mg/d may be indicated in combination therapy.
A recent study that analyzed the findings of 2 pivotal clinical trials concluded that the differences in efficacy between a regimen of 25mg/d and one of 50mg/d do not justify the risk of the adverse effects that occur with the higher dose, and the authors recommended 25mg/d for maintenance regimens.64,65
Since the pharmacokinetics, efficacy, and adverse reaction profile of acitretin are subject to interindividual variations, it is not possible to establish a standard dose. The regimen must, therefore, be tailored on a case-by-case basis by adjusting the dose to obtain the best clinical outcome with the lowest possible occurrence of adverse events.66
Although the SPC for Neotigason continues to specify that treatment with acitretin for more than 6 months is contraindicated, neither the experience of years of use nor results from several retrospective studies indicate any substantial increase in adverse effects with longer term therapy.
The recommended starting dosage for acitretin in plaque psoriasis is 10 to 20 mg/d. This initial regimen should be continued for 4 weeks. Response to treatment should be assessed at 4 weeks, after which the dose should be gradually increased to achieve the best therapeutic effect with the fewest adverse effects (minimal effective dose). In general, the maintenance regimen in most series varies between 25 and 50mg/d. Psoriatic lesions clear faster when the starting dose is higher, but such high-dose regimens also increase the risk of mucocutaneous adverse effects and consequently the risk of the patient stopping treatment due to poor tolerance of side effects. Initiating treatment at a high dosage (1mg/kg/d) is currently only recommended for the treatment of generalized flares of pustular psoriasis.
In some patients, psoriasis may worsen after the start of treatment, a phenomenon that manifests as more intense erythema and a greater spread of lesions, which later resolve.61
EfficacyAcitretin has be shown to be an effective treatment for psoriasis in both monotherapy and combination regimens.34–36,45,56,67 Accumulated experience with the drug and studies of its efficacy situate it in the group of less effective systemic antipsoriatic drugs, although no trials have directly compared acitretin with other antipsoriatic agents. The efficacy of acitretin, and most of its adverse effects, are dose-dependent and vary considerably from patient to patient. It usually takes 3 to 6 months to achieve maximum response.38,53,61,62,68,69
Plaque PsoriasisVery few studies have evaluated the short-term efficacy of acitretin in plaque psoriasis. A study that examined efficacy at 8 weeks in patients who received an initial dose of 10, 25, or 50mg of acitretin reported a mean reduction in PASI of 61%, 79%, and 86%, respectively, in the 3 treatment groups as compared to 30% in the placebo group.54
The available studies on the efficacy of the drug have very varied designs and objectives. Overall, initial doses of between 40 and 50mg/d have achieved a 50% reduction in PASI (PASI 50) in 66% to 85% of patients and a 75% reduction (PASI 75) in 34% to 52% of patients at 8 to 12 weeks. Higher dose regimens—up to 70mg/d—were associated with adverse effects that make it difficult for patients to continue treatment.36,45,67,70
Clinical experience has shown acitretin to be an ideal choice for long-term maintenance therapy because the clinical response is sustained and there is no significant loss of efficacy over time. The authors of a study that enrolled 63 patients in which efficacy was assessed following 12 months of treatment with acitretin reported that, of the 37 patients who completed the study, 89% had a PASI 50 response and 78.4% a PASI 75 response.36
Pustular PsoriasisIn generalized pustular psoriasis, acitretin is used at a dose of 0.5 to 1mg/kg/d and treatment generally produces a rapid clinical response with clearing of the lesions within 10 days. The dose should then be tapered slowly until a maintenance dose of 10mg/d is reached. Relapse on withdrawal of treatment is rare, and in such cases reintroduction of the drug restores the same efficacy. Acitretin is probably the first-line therapy in this form of psoriasis, with studies showing it to be effective in 84% of patients.50,71
It is also considered to be among the first-line options for palmoplantar pustulosis, either in monotherapy or in combination with phototherapy.72
Nail PsoriasisNumerous isolated cases in the literature demonstrate the efficacy of acitretin in the treatment of nail psoriasis. In an open study of 36 patients with moderate to severe nail psoriasis treated with acitretin at a dose of 0.2 to 0.3mg/kg/d for 6 months, a good response was observed, with a mean reduction in the Nail Psoriasis Severity Index (NAPSI) score of 41%.73 Complete clearing of lesions was achieved in 9 patients (25%), and there was no improvement in 6 (11%). These findings prompted the authors to propose this low-dose regimen of acitretin as a therapeutic option for nail psoriasis because of its good tolerance profile and scant adverse effects. By contrast, no improvement was achieved in a recent open-label study of 30 patients with nail psoriasis treated with acitretin at 0.3mg/kg/d during the first month and 0.5mg/kg/d for a further 3 months.74
Toxicity and Adverse ReactionsOverviewMost of the adverse effects of acitretin are dose-dependent and, in general, well tolerated at the recommended doses. Adverse clinical reactions and laboratory abnormalities occur less often with low-dose regimens (25mg/d) than with high ones (50mg/d).65,68 However, the toxic dose of acitretin is very close to the therapeutic dose, and for this reason most patients experience some adverse effects during the initial period of treatment while the dose is being adjusted. Such effects are usually reversible and disappear when the dose is reduced or treatment is interrupted, although in some cases they lead to discontinuation of treatment.
The adverse effects most often associated with the use of acitretin are shown in Table 2.
Most Commonly Reported Adverse Effects Associated With Acitretin.
| Frequent | Very Frequent | Infrequent | Very infrequent |
| • Rhinitis | • Cheilitis | • Xerostomia | • Pancreatitis |
| • Xerosis | • Alopecia | • Epistaxis | • Exacerbation of inflammatory bowel disease |
| • Nail dystrophy | • Peeling | • Diffuse erythema | • Agranulocytosis |
| • Pruritus | • Paronychia | • Leukopenia | |
| • Hyperestesic skin | • Myopathy | ||
| • Sticky skin | • Pseudotumor cerebri | ||
| • Xerophthalmia | • Depression | ||
| • Arthralgia, myalgia | • Retinoic acid syndrome | ||
| • Hyperostosisa | • Reduced night vision | ||
| • Headache | • Toxic hepatitis | ||
| • Nausea | • Edema of the face and limbs | ||
| • Abdominal pain | • Erythema multiforme | ||
| • Hyperlipidemia | • Vasculitis | ||
| • Photosensitivity | • Galactorrhea, gynecomastia | ||
| • Pulmonary fibrosis | |||
| • Teratogenicityb |
The most common adverse effects associated with acitretin affect the skin and mucous membranes.75 The effects are dose dependent and related to decreased sebum production, thinning of the stratum corneum, and impaired skin barrier function. Dryness of the lips and cheilitis occur in over 75% of patients. The next most common mucocutaneous effects are skin exfoliation or peeling, which can affect any part of the body but especially the palms and soles (in up to 50% of patients), rhinitis and epistaxis caused by drying of the nasal mucosa (up to 30%), and generalized xerosis, including xerophthalmia and xerostomia (Neotigason SPC).76 Less common adverse effects include nail fragility, periungual pyogenic granuloma, and a condition called retinoid dermatitis, which presents as scaly erythematous plaques or follicular papules and/or vesicles, predominantly affecting the face, forearms, chest, flanks, and the dorsum of the hands. These lesions have the histologic features of nonspecific acute dermatitis77 and must be distinguished from a psoriasis flare.69 Some patients experience photosensitivity reactions due to thinning of the stratum corneum.
These adverse effects usually only require symptomatic treatment, combining hydration and topical corticosteroids, or a temporary reduction in the dose of acitretin.
During the initial weeks of treatment, an increase in hair loss may be observed, sometimes leading to transient diffuse alopecia caused by telogen effluvium (in up to 25% of patients). In such cases, a rapid decrease in the dose should be considered because it is otherwise unlikely that the patient will adhere to treatment. The rate of hair loss usually returns to normal within 6 to 8 weeks of the dose reduction. Changes in hair color and texture during treatment with acitretin have also been reported.78
Hepatic EffectsAcitretin is hepatotoxic when used in the long term. Elevations in aspartate alanine aminotransferase, aspartate aminotransferase, and lactate dehydrogenase are seen in one-third of patients. These abnormalities are usually transient or reversible upon reduction of the dose or discontinuation of treatment.76 If liver enzymes are persistently or excessively high, the possibility of acute toxic hepatitis should be investigated; this adverse effect is rare (0.26% of patients)66 and also reversible upon cessation of treatment. There is anecdotal evidence of fatal progression from acitretin-induced acute toxic hepatitis to cirrhosis.79
The hepatotoxicity of acitretin is exacerbated by alcohol intake, obesity, diabetes, and certain hepatotoxic drugs. Of these, alcohol may have the greatest impact since it directly influences and modifies the metabolism of retinoids and is thought to be the factor that maximizes the severity of acitretin-associated hepatotoxicity.80 Complete abstention from alcohol is therefore recommended during treatment with acitretin.
Metabolic EffectsOne of the most common adverse effects of acitretin therapy, together with muscosal alterations, is an abnormal lipid profile. An increase in triglycerides is observed in up to 66% of patients, hypercholesterolemia in 33%, and a decrease in high density lipoprotein in up to 40% of patients.81,82 The changes in lipid levels are dose-related. In general, triglycerides levels tend to rise to between 2 and 3 times the upper limit of normal, rarely reaching levels associated with a risk of pancreatitis or eruptive xanthomas.69,81,82
The risk of abnormal lipid levels and pancreatitis increases when there is a family history of hypertriglyceridemia, alcohol consumption, obesity, or diabetes.81,82
In diabetic patients, acitretin treatment can alter glucose tolerance and lead to a disruption of glycemic control. Blood sugar levels should therefore be checked frequently in these patients, especially during the initial weeks of treatment. However, no increased risk of nondiabetic patients developing diabetes has been reported.76
Neurologic Effects Pseudotumor CerebriIn rare cases, pseudotumor cerebri or benign intracranial hypertension has been associated with the use of systemic retinoids.83 This condition presents with headache, visual disturbance, nausea, and/or vomiting. Patients taking acitretin should be warned to contact their physicians if they experience any of these symptoms. An eye examination should be performed urgently and treatment with acitretin must be discontinued immediately if papilledema is observed.
Pseudotumor cerebri was first reported in association with acitretin combined with tetracycline or minocycline,82,84 but it is also possible with acitretin used in isolation.76
Ophthalmologic EffectsXerophthalmia and eye irritation are relatively common adverse effects and may require the use of artificial tears, particularly in older patients (physiological xerophthalmia) and contact lens users. In some cases, ocular dryness is very severe and precludes the use of contact lenses during treatment. Other, much less common, side effects include the loss of eyelashes and decreased night vision. The onset of diplopia or blurred vision may be caused by papilledema and indicates the need for immediate withdrawal of acitretin (Neotigason SPC).76
Musculoskeletal EffectsSustained treatment with acitretin is often associated with arthralgia and myalgia; these symptoms are usually mild and can be controlled with oral analgesics. It is recommended that patients on acitretin should avoid intense physical exercise to minimize the risk of such symptoms.
Although early follow-up studies of patients treated with etretinate and acitretin reported a possible association with diffuse idiopathic skeletal hyperostosis,85 clinical experience accumulated over the decades since acitretin was first used as an antipsoriatic treatment has demonstrated that this effect is very rare53 (less than 1% of patients) and is primarily related to exacerbation of preexisting hyperostosis.86 Acitretin is not associated with increased susceptibility to bone fractures.87 Based on current data and the accumulated clinical evidence, it no longer appears reasonable to link acitretin to increased bone risk of any kind.52,53,88 Thus, we do not recommend performing bone tests before or during treatment except when a history of bone disease makes this advisable.
Retinoic Acid SyndromeRetinoic acid syndrome is a potentially fatal complication associated with the treatment of acute promyelocytic leukemia with all-trans retinoic acid therapy.89,90 The clinical features of this syndrome are as follows: fever, pulmonary infiltrates associated with respiratory distress, pleural or pericardial effusion, unexplained weight gain, ascites, impaired myocardial contractility, renal or hepatic insufficiency, episodic hypotension and, in some cases, coma. They typically occur in association with an elevated white blood cell count. The syndrome usually develops 7 to 10 days after start of treatment and is treated with high-dose corticosteroids. Although it is rarely associated with the use of acitretin, retinoic acid syndrome must be taken into account because some cases have been reported.91–93
TeratogenicityAcitretin is highly teratogenic (category X in the US Food and Drug Administration [FDA] classification of fetal risk) and causes characteristic fetal malformations similar to those seen in hypervitaminosis A (retinoid embryopathy).94
The use of acitretin at any dose during pregnancy can cause spontaneous abortions and major fetal malformations, including meningomyelocele, meningoencephalocele, skeletal malformations, cardiovascular malformations, decreased cranial volume, facial dysmorphia, and malformations of the thymus. The risk is higher when the drug is administered between the third and sixth week of pregnancy.94,95
Acitretin should not be taken by women who are pregnant or wish to become pregnant during treatment or up to 2 years after cessation of therapy according to the European Medicines Agency (Neotigason SPC) or up to 3 years according to the FDA (Soriatane SPC). Nor should it be prescribed to any woman of childbearing potential unless the prescribing physician considers the patient to be capable of using effective contraceptive methods during and after treatment.
Psychiatric SymptomsIn 1997, the authors of a study on the safety of acitretin reported that its use was linked to depression in between 1% and 10% of cases (Soriatane SPC). However, the methodological shortcomings of that study and the fact that only 1 case—considered doubtful—of an association between acitretin and depression has been reported in the literature96 raise doubts as to whether such an association really exists or whether the depression experienced by these patients is in fact due to the skin disease for which the acitretin treatment is prescribed. Nevertheless, and despite the fact that the relationship between isotretinoin—another retinoid—and depression and suicide has not been demonstrated either, the FDA has obliged manufacturers to include a warning about the risk of depression and suicide in the package insert for acitretin.97
SafetyAcitretin is a safe drug when it is correctly prescribed to the appropriate patient at the appropriate dose and when the patient is followed up regularly and the potential risks associated with toxicity are avoided, especially pregnancy in women.66,69Tables 3 and 4 list the conditions required for the safe use of acitretin and the situations in which precautions are necessary.
Measures for Enhancing the Safety of Treatment with Acitretin.
| Appropriate candidate selection |
| Use of health screening protocol before treatment |
| Review of the summaries of product characteristics for concomitant medications |
| Precise prescribing after considering contraindications |
| Gradual escalation of dose (indicative sign=cheilitis) |
| Protocolized follow-up and monitoring |
| Patients must guarantee and commit to being available for follow up visits and periodic testing. |
| Good accessibility to health care personnel. |
Evaluation of the Use of Acitretin in Situations of Risk.
| Indicated | Risk/Benefit Assessment | Not Indicated | |
| Obesity | ✓ | ||
| Type 2 diabetes mellitus | ✓ | ||
| Metabolic syndrome/dyslipidemia | ✓ | ||
| Moderate to severe CHF | ✓ | ||
| Live vaccines | ✓ | ||
| Demyelinating disease | ✓ | ||
| Active granulomatous disease | ✓ | ||
| Pregnancy | ✓ | ||
| Lactation | ✓ | ||
| <18 y/>65 y | ✓ | ||
| At risk for infections | ✓ | ||
| HIV/AIDS | ✓ | ||
| Chronic liver disease | ✓ | ||
| Hepatitis B virus carrier (asymptomatic) | ✓ | ||
| Hepatitis C virus carrier (asymptomatic) | ✓ | ||
| Renal insufficiency | ✓ | ||
| Psoriatic arthritis | ✓ | ||
| Melanoma | ✓ | ||
| Lymphoreticular neoplasia | ✓ | ||
| Solid carcinoma | ✓ |
Abbreviations: AIDS, acquired immunodeficiency syndrome; CHF, congestive heart failure; HIV, human immunodeficiency virus.
Adapted from Strober et al.58
Before starting treatment, patients should be well informed about the most common side effects of the drug because these can quickly lead to poor adherence to treatment. They should also understand that the clinical response may take time to appear and the physician should explain why acitretin has been prescribed in preference to other possible treatments. Patients who are given complete information and involved in the choice of drug are more likely to adhere to treatment.
Women must be advised that they must avoid pregnancy during treatment with acitretin and for at least 2 to 3 years after cessation.
Patients must be instructed to moisturize their skin and mucous membranes regularly and to use sunscreen and sunglasses to filter out UV radiation.
They must be made aware that before taking any other medications they should inform the prescribing physician that they are taking acitretin; this precaution is essential to avoid toxicity (hepatotoxicity, hypervitaminosis A syndrome).
The need for regular follow-up and testing to ensure the safety of treatment and make long-term use of the drug possible must be discussed with patients, and they must commit to this program.
Baseline Screening and Follow-up During TreatmentBefore starting a patient on treatment with acitretin, the physician must obtain a complete medical history (past history, comorbidities, concomitant medication, physical examination, prior course of psoriasis, type of psoriasis, and prior medication. In women, pregnancy must be ruled out and a contraceptive regimen put in place that must continue for at least 2 years after cessation of treatment according to the European label and 3 years according to the US label. Baseline blood count and biochemistry should be obtained; explorations should include liver and kidney function and lipid profile.69
Liver function and lipid levels should be reassessed a few weeks after start of treatment. The exact timing of this assessment will depend on the patient's risk profile and whether he/she is taking concomitant medication that might increase hepatotoxicity or lipid abnormalities. In our opinion, the first assessment during treatment should take place between 4 and 8 weeks and the standard program of monitoring check-ups should be initiated thereafter. In standard clinical practice, alterations in liver function and lipid values are not very common and monitoring every 6 months is sufficient.
Regular radiographic investigation is not recommended except when there is evidence of bone toxicity.53
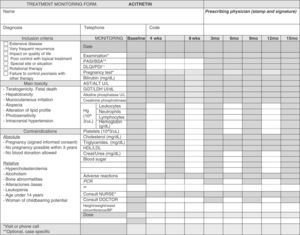
Clinical and laboratory parameters should be evaluated every 3 to 6 months (Table 5).69Figure 3 is a sample treatment monitoring form.
Screening of Candidates Suitable for Treatment with Acitretin.
| Medical history |
| Type of psoriasis |
| Course of disease |
| Arthitis/arthralgia |
| Response to prior treatments |
| Contraindications/risks |
| Baseline workup |
| Physical and dermatologic assessment |
| Liver function |
| Kidney function |
| Lipid profile |
| Blood sugar levels |
| Viral infections |
| Rule out pregnancy and prevent conception |
| Follow-up monitoring |
| Repeat physical and dermatologic assessment |
| Liver function |
| Kidney function |
| Lipid profile |
| Blood sugar levels |
| Easy and direct line of contact with the dermatology nursing staff |
The decision to use acitretin in patients with cardiovascular risk must be very carefully weighed because of the associated risk of hypertriglyceridemia and the consequent increased risk of atherosclerosis. If acitretin is prescribed, it is always advisable to add lipid-lowering therapy98 and to advise the patient to begin or further enhance a lifestyle designed to reduce cardiovascular risk factors.69
The use of acitretin in patients with metabolic syndrome must be considered on a case-by-case basis because of the risk of exacerbating the syndrome and increasing cardiovascular risk, and because the liver toxicity associated with acitretin is favored by obesity and diabetes. If the patient's lipid levels are affected, the dose should be reduced and lipid-lowering treatment should be added; in some cases, withdrawal of acitretin might even be considered.
Hypertriglyceridemia and hyperglycemia can be managed by increasing physical exercise, introducing dietary changes, reducing alcohol intake, and pharmacotherapy.
Increased efficacy of acitretin treatment has been reported in patients with type 2 diabetes mellitus taking pyoglitazone.99 This may be a result of the anti-inflammatory and antiangiogenic effects of this oral hypoglycemic agent.100 Pyoglitazone is a second-line oral antidiabetic agent and a first-line treatment in obese patients who cannot be treated with metformin. The prescription of this combination in a patient with psoriasis should involve an endocrinologist. It may occasionally be useful to take advantage of this synergistic effect in selected patients who have both psoriasis and diabetes.
Use in Patients With Impaired Liver FunctionSince acitretin is potentially hepatotoxic, it is relatively contraindicated in patients with hepatic impairment. If acitretin is used in such patients, liver enzymes must be monitored and the dose and regimen adjusted accordingly; alanine aminotransferase values should not exceed 4 to 5 times the upper limit of normal.
Liver biopsy is never required since no association has been found between cumulative doses of acitretin and fibrosis or cirrhosis of the liver.101 Habitual alcohol consumption, obesity, and diabetes exacerbate the hepatotoxic effect of acitretin and therefore increase the risk of such toxicity. In such cases, closer monitoring of liver function is required or the use of acitretin may be clearly contraindicated.
Use in Patients With Renal ImpairmentAcitretin is also contraindicated in patients with renal impairment (Neotigason SPC). Plasma concentrations of acitretin are lower in patients with severe kidney failure. Note that acitretin is not eliminated by dialysis.
Drug InteractionsLogically, the concomitant use of acitretin with other drugs or substances having similar adverse effects can exacerbate these effects, or the action of acitretin may alter the function of other drugs taken concomitantly. Whenever acitretin is prescribed, all the drugs taken by the patient before and during treatment must be recorded.
It is important to note that alcohol intake increases the hepatotoxicity of acitretin and favors the re-esterification of acitretin to etretinate, with the increased risk of teratogenicity that this implies (Neotigason SPC).76 It is also important to note that acitretin reduces the anticontraceptive effect of drugs based on microdoses of progestogens and that these minipills should never be used to prevent pregnancy during treatment. Contraceptives that use a combination of estrogen and progesterone may be used since acitretin does not interfere with the action of these drugs.102 The most important drug interactions are shown in Table 6.
Drug Interactions With Acitretin.
| Alcohol |
| Increases hepatotoxicity |
| Increases re-esterification of acitretin to etretinate (teratogenicity) |
| Tetracyclines |
| Increases photosensitivity |
| Risk of intracranial hypertension |
| Minocycline |
| Risk of intracranial hypertension |
| Vitamin A (>4,000-5,000 IU/d) |
| Hypervitaminosis |
| Corticosteroids |
| Altered lipid levels |
| Risk of intracranial hypertension |
| Methotrexate |
| Potential hepatotoxicity |
| Raises methotrexate levels |
| Ciclosporin |
| Alteration of lipid profile |
| Increases cardiovascular risk |
| Phenytoin |
| Increases blood levels of phenytoin |
| Oral antidiabetic agents |
| Fluctuations in blood sugar levels |
| Glibenclamide |
| Reduces hypoglycemic effect of glibenclamide |
| Progestogen contraceptives (in mini doses) |
| Reduces contraceptive effect |
Since acitretin is not a direct immunosuppressant it can be used in patients who are going to be vaccinated, whether with toxoids or with live, attenuated vaccines.
Use in Patients With Cancer and Immunocompromised PatientsAcitretin is a psoriasis treatment suitable for use in patients with cancer or in immunocompromised patients because it is not an immunosuppressant and because retinoids have been shown to have a chemopreventive effect in promyelocytic leukemia,103 precancerous and cancerous skin lesions,104–106 and hepatocellular carcinoma.107 Consequently, acitretin may be indicated as a first-line systemic treatment in patients with psoriasis who also have melanoma, solid tumors, or lymphoproliferative disorders,58 and in patients with human immunodeficiency virus infection.57,58
Use in Pregnant Women and Reproductive ConsiderationsThe use of acitretin during pregnancy is contraindicated because of the drug's teratogenicity and the risk that it may cause severe fetal malformations (independent of dose and timing of exposure).76
When acitretin is administered to woman of childbearing potential, contraception must start at least 1 month before start of treatment and must be maintained for 2 to 3 years after cessation of treatment. The minimum duration of contraception after cessation of treatment is specified as 2 years in the European SPC. The duration of this safety period has been calculated on the basis of evidence that 98% of the etretinate that may have been formed from the acitretin administered will be eliminated within 2 years. The 3-year contraception period after discontinuation of treatment specified in the US SPC (1 year longer than the calculation based on the half-life of the drug) has been established to increase the margin of safety, but is without pharmacologic basis.
If acitretin is prescribed to women of reproductive potential, it is essential to ensure adequate contraception from at least 1 month before start of treatment throughout the entire period of treatment, and for at least 2 years after cessation of treatment. Contraceptive measures must also be adopted if the patient receives repeated cycles of treatment. These precautions must be taken even by women who normally do not use contraception because of a history of infertility. Alcohol consumption is strictly prohibited in women of childbearing age during treatment and for 2 months following withdrawal of treatment. In such cases, convincing evidence that the patient understands and accepts the potential risks of treatment is essential and the patient must sign informed consent authorizing the use of acitretin.
Patients receiving acitretin must not donate blood during treatment or for 1 year following withdrawal of treatment to avoid all possible risk of teratogenicity in the eventual recipient of the donated blood. Nevertheless, a recent study that monitored the pregnancies of 18 women who accidentally received blood transfusions from individuals taking acitretin did not observe any fetal malformations or abnormalities during pregnancy.108
In male patients, acitretin has not been associated with abnormalities in spermatogenesis or the characteristics of spermatozoids.109 No cases of fetal malformation have been reported in pregnancies following conception while the father was taking acitretin. The presence of acitretin metabolites in the seminal fluid of men being treated with acitretin appears to entail very little risk for the fetus. However, the data available are scant and acitretin therapy is usually avoided in males planning to have children.110
Use in Breastfeeding MothersThe SPC contraindicates the use of acitretin in lactating mothers even though the quantities of drug excreted in maternal milk and the quantity that may be ingested by the infant are very small.48 This contraindication is based on initial concerns about the possibility of acitretin causing skeletal abnormalities and the results of animal studies performed at very high doses in which abnormalities were observed in the diaphyseal and spongy bone of offspring. However, since the risks of bone abnormalities have been minimized and relativized over time and the doses used in the studies in question were much higher than those recommended for use in humans, acitretin can be used in nursing mothers after careful consideration of the risk-benefit ratio in each case.111
Pediatric UseThe use of acitretin in children is also contraindicated based on data relating to long-term treatment with etretinate in childhood in which skeletal alterations were reported, including premature epiphyseal closure, skeletal hyperostosis, and extraosseus calcification. Based on the assumption that such changes could also appear in children taking acitretin—although this has not been demonstrated—the SPC for Neotigason advises against the use of acitretin in children. However, in light of the evidence available after nearly 2 decades of use, we believe that this contraindication is relative and that acitretin can be prescribed in specific cases as long as the risk-benefit ratio is favorable.
In children, the dose of acitretin is weight-adjusted and the lowest possible dose is determined by the commercial availability of the drug in 10mg doses. The recommended starting dosage is 0.5mg/kg/d and this is escalated gradually taking into account the clinical response and the appearance of adverse effects. Doses in excess of 35mg/d are only recommended in exceptional cases (Neotigason SPC). Once a clinical response is achieved, the dose should be reduced to the lowest effective maintenance regimen to avoid, as far as possible, long-term adverse effects.
Use in Elderly PatientsThe indications and regimens for the use of acitretin in older patients are the same as for adults in general. However, it should be remembered that older patients are more susceptible to cutaneous and mucosal xerosis and liver and kidney failure. Consequently, these adverse effects may appear at lower doses in these patients. The doses used should therefore be lower, particularly at the start of treatment, and these patients should be more closely monitored.
Use in Patients Undergoing Surgical InterventionsThe use of acitretin is not contraindicated in patients undergoing surgery, although the physician should carefully consider the possible benefits of reducing the dose or even temporarily suspending treatment with the drug to prevent possible postoperative complications associated with its adverse effects (Table 2).
OverdoseThe effects of accidental or intentional overdose with acitretin are similar to the manifestations of acute vitamin A toxicity, namely, severe headache, nausea or vomiting, drowsiness, irritability, and pruritus. These symptoms subside without treatment. Owing to the variable absorption of the drug, gastric lavage may be indicated in the initial hours following an overdose (Neotigason SPC).
Treatment Strategies for Acitretin in PsoriasisInitial Dose Followed by Gradual EscalationApproaches that seek to achieve a rapid response at start of treatment with acitretin are generally counterproductive because they lead to the appearance of adverse effects, which eventually limit the use of the drug.38 The primary aim should be to identify the dose that each patient can tolerate and to maintain this regimen while waiting for a clinical response in the long term (the therapeutic objective should be assessed 3 to 6 months after start of treatment).
Although the usual therapeutic dosage of acitretin is between 25 and 50mg/d, it is preferable to start treatment with a lower dosage (between 10 and 25mg/d)61 and then tentatively raise the dose by 10mg every 2 weeks until the adverse effects experienced by the patient make it advisable to refrain from further escalation.53,65 A useful sign is the appearance of cheilitis, which is an expression of the correct bioavailability of acitretin in each individual.16 Note that the individual variability associated with this drug means that low doses of acitretin may be effective.64 Thus, gradual dose escalation is the optimal strategy for the correct use of this drug since this approach makes it possible to obtain the best clinical response with the least adverse effects.68 The escalation approach also allows patients to gradually increase their tolerance of the potential and frequent adverse effects associated with acitretin and avoids the use of higher-than-necessary doses in some cases or doses that might lead to discontinuation of treatment in others.53,61
Gradual dose escalation strategies take time, and attempts to shorten this time generally result in discontinuation of treatment before its long-term efficacy has been demonstrated. This gradual approach, which is the key to the correct management of therapy with acitretin in clinical practice, contrasts sharply with the strategies and responses we are accustomed to with other antipsoriatic drugs, some of which achieve dramatic results in a relatively short period.
Adjusted Dose and Maintenance RegimenOnce the optimal dose has been achieved using the escalation strategy, and if an acceptable clinical response is achieved, it is normally possible to slightly reduce the dose to improve tolerance. Once again, this requires individualized dose adjustment achieved by gradually tapering the dose. Using this strategy it is often possible to maintain efficacy with low-dose regimens (10 to 25mg/d; or 25mg/48h) for a very long period.66
Combination TherapyThere is evidence supporting the usefulness of combining acitretin with topical treatment, phototherapy, classic systemic therapies, and biologic agents, either as an initial drug to which a second treatment is added or as a second drug added to an already established regimen.112,113
A recent study that analyzed the data on the prescription of acitretin recorded in the National Ambulatory Medical Care Survey found that in 62% of cases acitretin was prescribed concomitantly with another drug: topical corticosteroids (51%), calcipotriol (31%), biologic agents (6%), ciclosporin (5%), methotrexate (5%), and tazarotene (2%).114Table 7 shows the different options for combination therapy with acitretin.
Classic Acitretin Combination Strategies.
| Starting with Acitretin and Adding a Second Drug |
| Acitretin+methotrexate |
| Full doses of both drugs can be administered |
| Acitretin may be withdrawn abruptly |
| Monitor hepatotoxicity |
| Acitretin+ciclosporin |
| Full doses of the both drugs can be administered |
| Acitretin can be withdrawn abruptly |
| Monitor lipid profile |
| Acitretin+phototherapy |
| Acitretin at normal doses, reduce phototherapy dose by 50% |
| Discontinue acitretin when clearing is achieved |
| Starting with Another Drug, Adding Acitretin |
| Methotrexate+acitretin |
| Taper dosage of methotrexate over 2-3 mo to 7.5mg/wk |
| Introduce acitretin at full dose |
| Monitor hepatotoxicity |
| Ciclosporin+acitretin |
| Full dose of ciclosporin |
| Add acitretin at low daily doses (or every 48 h) |
| Gradually increase dose of acitretin, depending on the clinical response |
| Once response has been obtained, taper dose of ciclosporin over 3-4 months |
| Maintain acitretin therapy |
| Monitor alterations in lipid profile |
| Phototherapy+acitretin |
| Reduce phototherapy dose to 50% a week before introducing acitretin |
| Add acitretin at 10-25mg/d |
| Gradually increase phototherapy dose according to response and phototoxicity |
| Increase acitretin to an acceptable dose (most common maximum dosage is 25mg/d) |
| Maintain acitretin and phototherapy until clearing is achieved |
| Biologics+acitretin |
| Maintain dose of tumor necrosis factor inhibitor |
| Add acitretin at a low dose |
| Gradually escalate dose of acitretin according to response |
Adapted from Lebwohl et al.112
The therapeutic efficacy of acitretin increases when it is combined with topical calcipotriol70,115,116 or topical corticosteroids.112,117 The combination with calcipotriol (1,25-dihydroxyvitamin D3) is of particular interest since this drug also induces the transcription of certain genes.28,118,119
Use in Combination With PhototherapyThe association of oral retinoids and phototherapy is probably the most well-known and generally accepted combination therapy. The benefit of this combination was initially demonstrated with etretinate and PUVA therapy120,121 and, given the clinical equivalence of the 2 drugs, the results obtained with etretinate may be extrapolated to acitretin. Moreover, the combination of acitretin with UV-B, UV-A, or PUVA therapy has also been shown to be more effective than monotherapy with acitretin or phototherapy the use of a combination makes it possible to reduce the number of phototherapy sessions and the dose of acitretin required, thereby improving tolerance.52,66,122–124 Moreover the anticarcinogenic potential of the retinoids makes combination treatment safer than phototherapy alone.
When clearing is used as a measure of efficacy, the result achieved with acitretin in combination with UV-B therapy (74%) is good and better than that achieved by monotherapy with either acitretin (42%) or UV-B (35% to 41%).66,125,126 The acitretin–PUVA combination has also been shown to be more effective than monotherapy with either treatment.127 The acitretin–UV-B and acitretin–PUVA combinations have similar efficacy in the treatment of psoriasis.128 UV-B, and particularly narrowband UV-B, is currently preferred to PUVA in combinations with acitretin because it does not produce the adverse effects associated with psoralens.
When combination therapy is chosen from the outset, the recommended strategy is to administer acitretin at a low dose (for example 25mg/d) for 2 weeks before starting phototherapy. When the patient is already receiving UV-B phototherapy but the therapeutic objective has not been achieved, 25mg/d of acitretin may be prescribed. In such cases the dose of UV-B should be reduced by between 30% and 50% during the first week to prevent erythema. It is then gradually increased until clinical response is achieved with acceptable tolerance.69 Once the lesions have cleared, the dose of acitretin should be maintained for 1 month before reducing either the dose of acitretin or the frequency of phototherapy sessions.129
Use in Combination With Classic Systemic TreatmentsThe association of acitretin and the immunosuppressive agents used to treat psoriasis is supported only by evidence from isolated case reports and series. Acitretin is the only systemic antipsoriatic agent that can be used in combination with another systemic drug without creating a regimen of 2 immunosuppressants, and consequently the only one that will not increase the risks arising from immunodeficiency.
The combination of acitretin with methotrexate is effective,130 particularly in the case of pustular psoriasis.131 If, after starting acitretin at the usual doses a therapeutic response is not achieved within the established time frame (normally 3 to 6 months), weekly administration of methotrexate may be added at the usual doses. Once clinical improvement has been achieved, 1 of the 2 drugs may be discontinued130 or the combination can be maintained at lower doses for both drugs.
Given that both acitretin and methotrexate are hepatotoxic, close monitoring of liver function is essential. However, the concern about hepatotoxicity that initially motivated the formal contraindication to this combination in the Neotigason SPC is not usually a problem in clinical practice in our experience and in that of other authors,112 and tolerance is generally good.132
The combination of acitretin and ciclosporin has also been used effectively in the treatment of psoriasis, although it is debatable whether it offers any advantage over monotherapy in terms of efficacy.133,134 In our clinical experience we have not observed any such advantage. Furthermore, prolonged use of this combination increases the risk of elevating cholesterol and triglyceride levels and of potentiating the toxicity of ciclosporin because acitretin inhibits the P-450 cytochrome, which is the principal inactivation pathway of ciclosporin. The combined use of acitretin and ciclosporin is more useful as a temporary overlap in the context of a sequential strategy (see below), or in selected cases of psoriatic erythroderma.135,136
Use in Combination with Biologic TherapyThe combination of acitretin with the new selective immunosuppressants (biologics) is also a treatment option, especially in cases in which the biologic treatment has not achieved complete control of the disease and in which the antipsoriatic, but not immunosuppressive, action of acitretin may add the small degree of efficacy required to completely control the disease. Examples of such cases are patients with inflammatory bowel disease and psoriasis, patients with generalized psoriasis in whom therapy has not completely cleared lesions on the palms and soles, and patients with psoriasis previously treated with PUVA and ciclosporin who are on biologic treatment to reduce the risk of nonmelanoma skin cancer associated with immunosuppression.137–139
A series of cases have recently been published of patients who responded to combinations of biologic drugs with acitretin.137,138,140,141 In these patients, the efficacy of monotherapy with a biologic agent was suboptimal and the addition of acitretin improved efficacy. These findings suggest that efficacy can be improved with no increase in toxicity, since acitretin is not an immunosuppressant. Furthermore, by using acitretin, physicians can reduce the dose of the biologic while maintaining response, enhancing safety, and above all reducing costs.
A recent open-label retrospective study assessed the addition of a biologic agent in a series of patients who had a poor response to acitretin therapy.138 As might be expected, the regimen was shown to be effective and is an example of the role acitretin can play in the era of biologic therapy. Because of its proven efficacy and very good long-term safety profile, acitretin may be another option to consider when choosing a combination strategy when a biologic drug does not achieve optimal efficacy or when response to biologic therapy diminishes. Moreover, acitretin is distinguished from ciclosporin, methotrexate, and the biologics in that its mechanism of action is not based on immunosuppression. To date, the tendency, perhaps influenced by the practice of rheumatologists, has been to use methotrexate with biologics as the first-line combination therapy, even in the absence of arthritis.
Sequential TherapySequential therapy has been established as a strategy for the long-term management of psoriasis to avoid the prolonged use of immunosuppressants and drugs with cumulative, organ-specific toxicity. The approach involves using a different drug as a maintenance regimen after the therapeutic objective has been achieved with the induction treatment; it can be a drug-sparing option in the long-term.16,142
Acitretin is an ideal candidate for sequential strategies because of its capacity to maintain efficacy in the long term without direct immunosuppressive action. Moreover, in sequential approaches the slow response associated with acitretin treatment can be compensated for by starting treatment with a faster-acting and more effective antipsoriatic drug whose long-term use is limited by the risk of exceeding a maximum cumulative dose or even by financial considerations. The idea is that once improvement has been achieved with one treatment, it can be maintained using acitretin, preferably at low doses.66,143
A classic sequential strategy involves using ciclosporin to clear the patient's psoriasis (clearing phase) and then adding a gradually escalating dose of acitretin (transition phase) to establish the maximum tolerated dose. The last step (maintenance phase) is to discontinue ciclosporin and maintain long-term monotherapy with acitretin.142 Another variant of this strategy is to occasionally add UV-B or PUVA therapy in the case of a loss of response to monotherapy with acitretin (Table 8).66
Sequential Therapy with Ciclosporin and Acitretin.
| Phase 1 |
| Induction with ciclosporin |
| Start ciclosporin at 5mg/kg/d |
| Continue with ciclosporin at 5mg/kg/d |
| Phase 2A |
| Achieve clearing |
| Introduce acitretin at a low dose and escalate gradually to the maximum tolerated dose |
| Phase 2B |
| Maintain clearing (>6-7 mo) |
| Gradually taper ciclosporin to <1mg/kg/d |
| Maintain acitretin dose |
| Phase 3A |
| Consolidate clearing with acitretin monotherapy |
| Withdraw ciclosporin |
| Maintain treatment with acitretin |
| Phase 3B |
| Long-term clearing Adjustment for fluctuations |
| Maintain acitretin dose |
| Add phototherapy if required |
Sudden interruption of acitretin therapy does not produce a rebound effect resulting in a flare.144 This means that treatment with acitretin, whether as monotherapy or combination therapy, can be suspended at any time. Sustained remission of psoriasis after a prolonged period with therapeutic response can last 2 to 6 months.
Because of these characteristics and the absence of any known antigen action or resistance, as long as a rapid clinical response is not required, acitretin is suitable for intermittent therapy, although this type of treatment regimen has not been assessed in clinical trials
ConclusionsAcitretin is a second-generation synthetic retinoid that is effective in the systemic treatment of psoriasis, either in monotherapy or in combination or sequential regimens. Although it has a much shorter half-life than etretinate and does not accumulate in any particular tissue, its use is nonetheless contraindicated in pregnant women and women of childbearing age who plan to conceive within at least 2 years of cessation of treatment.
Acitretin's efficacy in the treatment of psoriasis is mediated by the transcription and inhibition of specific genes, through which it influences the action of intracellular retinoids and exerts a primarily regulatory effect on the differentiation and proliferation of keratinocytes and, to a lesser degree, acts as an anti-inflammatory.
Compared to other available systemic drugs (methotrexate, ciclosporin, and biologics), acitretin is less effective, but it nonetheless achieves significant clearing and can be used in the long term without risk of immunosuppression.
In monotherapy, acitretin obtains a therapeutic response (PASI 75) in 40% to 50% of patients. The best results are achieved when the dose is gradually escalated to achieve the maximally effective dose, which varies in each individual.
Acitretin is especially useful in the treatment of pustular psoriasis and the palmar and plantar forms of the disease. Its use in combination with phototherapy or in sequential therapy with ciclosporin is also of particular interest. When used in association with biologic drugs it offers a strategic advantage in the long-term management of psoriasis. Since it is not a direct immunosuppressant, acitretin can be used in patients in whom the risk of immunosuppression may limit the use of other antipsoriatic drugs.
While it does not have a rapid onset of action, the value of acitretin in patients with psoriasis lies in its potential for very prolonged use as maintenance therapy after clearing has been achieved, or as a component of combination regimens.
In summary, although acitretin is not usually a first-line option for clearing moderate to severe psoriasis, it should be considered in the overall long-term management of psoriasis as a maintenance treatment to prevent relapse; it can be very useful in specific circumstances and in certain clinical forms of the disease. The management of acitretin therapy may be more complicated than that of other drugs, but when used correctly it remains useful in the treatment of psoriasis even in the era of biologic therapies.2,4Table 9 summarizes the use of acitretin in the treatment of psoriasis.
Use of Acitretin in Psoriasis.
| Indications |
| Generalized pustular psoriasis and palmoplantar psoriasis |
| Psoriatic erythroderma |
| Psoriasis vulgaris (single-drug, combination, and sequential therapy) |
| Contraindications |
| Absolute: pregnancy, allergy to any of its components, severe hepatic or renal impairment |
| Relative: rental or hepatic impairment, metabolic syndrome |
| Mechanism of Action |
| Genetic transduction through the activation of specific nuclear receptors |
| Acts on keratinization, cell proliferation, and inflammation |
| Immune modulation |
| Pharmacology |
| Half-life of 50h (its metabolite 13-cis-acitretin 90h; and etretinate 120d) |
| Does not accumulate in tissue |
| Renal and fecal excretion via bile |
| Is converted to etretinate by re-esterification (accelerated by alcohol) |
| Efficacy |
| Slow response |
| Assess achievement of therapeutic objective at 3-6 mo |
| Short term (12 wks): PASI 50 in 80%/PASI 75 in 52% |
| Long-term (12 mo) PASI 50 in 89%/PASI 75 in 78% |
| Dosing |
| The initial dosage of acitretin should be between 10 and 25mg/d |
| Dose increase every other week (indicative sign: appearance of cheilitis or xerosis) |
| The optimal dosage is between 25 and 50mg/d |
| Once the desired clinical response has been achieved, consider gradually reducing the dose until the minimum effective dosage is achieved. This can then be maintained for long periods (10-25mg/24h, 25mg/48h) |
| Toxicity |
| Severe: teratogenicity, hepatotoxicity, dyslipidemia (cardiovascular risk) |
| Mild: mucositis, xerosis, alopecia, laminar scaling, myalgia, photosensitivity, epistaxis, paronychia, nausea, abdominal pain, sticky skin (see Table 3) |
| Safety |
| Ensure correct patient screening and selection |
| Patient information and education |
| Use dose in therapeutic range |
| Individualized dose adjustment guided by the appearance of adverse effects |
| Strategies |
| Monotherapy |
| Combination therapy |
| Sequential therapy (ciclosporin sparing) |
| Combinations |
| Acitretin+phototherapy |
| Acitretin+methotrexate |
| Acitretin+biologic agent |
| Acitretin+topical calcipotriol |
| Screening and Monitoring |
| Baseline physical examination and medical history |
| Rule out pregnancy (fertile women) |
| Identification of contraindications and comorbidities at baseline and regularly thereafter |
| Check the list of concomitant medications the patient is taking |
| Baseline and regular laboratory workup according to protocol (see attached sample treatment monitoring form, for example) |
The authors deem that they have no conflicts of interest with respect to these guidelines. However, all those who participated in drawing up these guidelines have had and continue to have, to differing degrees, relationships with the pharmaceutical industry and have received financial support for conference attendance, clinical trials, or their work as scientific research consultants in relation to other antipsoriatic drugs.
Please cite this article as: Carretero G, et al. Acitretina: guía de uso en psoriasis. Actas Dermosifiliogr. 2013;104:598–616.